nerve activation - diglet48/restim GitHub Wiki
We will discuss:
- Basic theory on nerve activation.
- Test setup and measurements for subjective intensity of different pulse widths.
- The relationship between signal intensity, pulse width and amplitude.
- Energy efficiency.
- Electrode size.
- Magnitude scaling.
- Monophasic pulse integration.
- Muscle vs sensory nerve stimulation.
- Pulse shape.
- Skin depth.
- Selective activation.
Theory
A cell maintains a potential relative to the medium it's floating in, the resting potential. The potential can be adjusted by injecting charge in the cell, once the potential reaches the activation threshold an action-potential is generated. For short pulses, the charge (in Coulomb) injected in the cell is the fundamental quantity defining stimulus potential.
While charge is being injected in the cell, the cell works to restore the charge balance back to neutral. This called the cell leakage. The slower we inject change (longer pulse width), the more charge is lost. As a result wider pulses need to inject more charge to reach activation threshold.

Below I will perform an experiment to measure the leakage. With this information, we can easily generate narrow and wide pulses with the same subjective intensity.
Once an action-potential is generated, the cell needs some rest time. Activating various nerve cells over a certain time period can result in a specific sensation. This is not investigated further on this page.
Test setup and basic equations
I used a stereostim box with oscilloscope attached. 2 3x3cm conductive rubber sheets attached to myself. Pulsed sine wave 5 carrier cycles at 50hz pulse frequency.
The pulsetrain consisted of 20 pulses of a control signal followed by 20 pulses of the test signal. The test signal was of medium-low intensity. I controlled the volume of the test signal until both signals felt equally strong.
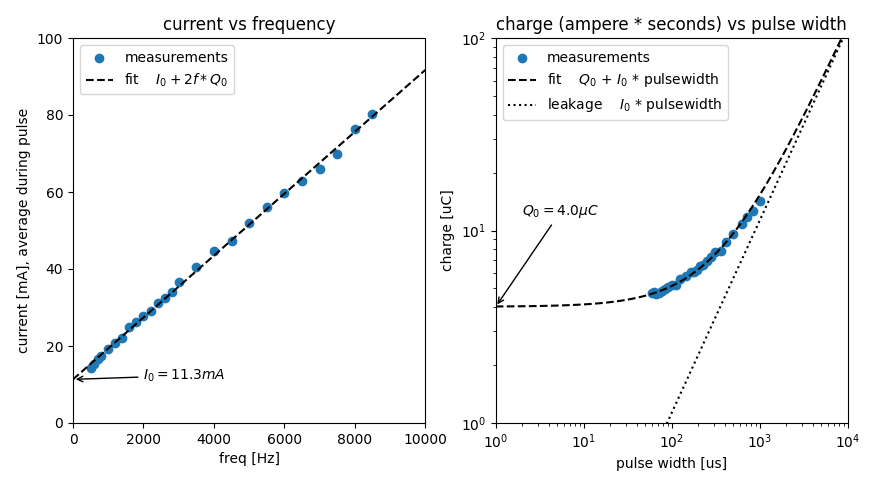
Left pane contains the test data with a linear fit. Right pane shows the same data with slightly different units. The pulse width is $\frac{1}{2*f}$.
Three symbols are of note:
$I_0 = 11.3mA$, the rheobase, this is the minimum current to elicit this nerve response for a very wide pulse.
$Q_0 = 4.0\mu C$, the minimum charge to elicit this nerve response for a very narrow pulse.
$\tau = \frac{Q_0}{I_0} = 355\mu s$, the chronaxie, the time constant of the nerves.
$I_0$ and $Q_0$ are measures of nerve activation, or subjective signal strength. I prefer using $Q_0$.
The value of $\tau$ isn't a constant, but depends a bit on nerve type, electrode placement, and such. But for most purposes a constant is a pretty good approximation.
To estimate the value of $\tau$ you don't need to trace the entire strength-duration curve, only data from one short pulse and one long pulse is needed.
Some sources calculate $\tau$ from the minimum voltage instead of current, I think that's a terrible idea.
Some useful equations
$I_T$ and $Q_T$ indicate the signal amplitude and total charge in the pulse (not taking into account the cell leakage). These are called the threshold current and charge.
$I_T = I_0 * (1 + \frac{\tau}{pulsewidth})$
$Q_T = Q_0 * (1 + \frac{pulsewidth}{\tau})$
Maximum charge for sine wave: $\frac{\text{current amplitude}}{\text{frequency} * \pi * 2}$ (charge balance oscillates between -charge and +charge).
If you want to generate two pulses at different pulse widths but same subjective intensity, you can use this formula.
$amplitude_1 * (\frac{\tau}{pulsewidth_2} + 1) = amplitude_2 * (\frac{\tau}{pulsewidth_1} + 1)$
Some numbers on pulse intensity
I gathered these numbers during experiments with various size electrodes.
| Effect | Charge ($Q_0$) |
|---|---|
| detection threshold | $0.5\mu C$ |
| medium strength sensation | $2\mu C$ |
| cum | $3 - 4\mu C$ |
| Pain tolerance | $4 - 7\mu C$ |
| intense muscle contractionsfor internal electrodes | $2.5 - 3\mu C$ |
Energy efficiency
Literature (ref 1, equation 4.10 and 4.11) says the stimulus energy reaches a minimum when the pulse width is $1.25\tau$. This does not match my measurements. The image below shows the power (in watt) for pulsetrains used in the experiment at the beginning of this page.
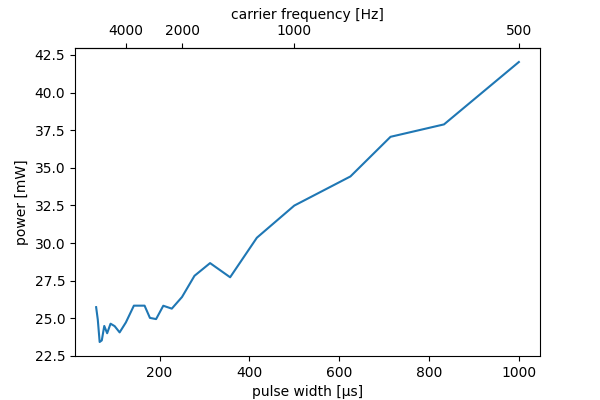
I think literature used measurements on a single isolated nerve, but my tests are performed on skin. Skin resistance decreases as the frequency increases. As a result my numbers differ.
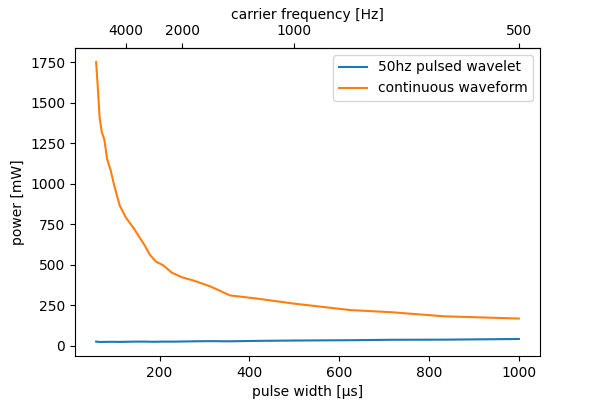
The improved energy efficiency with higher frequencies only exists with pulsed waveforms. The figure below compares the energy usage of wavelets with those of continuous signals (approximate, signal intensity not exactly equal). Be extremely careful with high frequency continuous signals as you can easily burn yourself.
Electrode size
For electrodes smaller than $1\text{mm}^2$ the signal intensity does not depend on the electrode area.
For larger electrodes, perception/pain tolerance threshold follows a power-law relation with the electrode area. The scaling is approx 1/6 for finger and 1/3 for arm. (ref 1, table 7.7).
If we assume finger stimulation, doubling the area requires $2^\frac{1}{6} = 12\%$ more charge for the same signal intensity. In other words, $44\%$ lower current density. This suggests that current or charge is a better measure of signal intensity than current density.
Due to the relatively weak relationship between area and signal intensity, you don't need to match the size of your electrodes.
Magnitude scaling
Subjective signal intensity (M) following a stimulus (I) is in the form of:
$M = \alpha * I^\beta$
$\alpha$ is a scaling constant and $\beta$ indicates rate of growth. $\beta$ differs per body part and nerve type, values between 1.5 (leg) and 2.5 (finger) have been observed in literature. (ref 1 figure 7.7).
Monophasic pulse integration
The cell potential is effectively the integral of the signal over the time constant $\tau$. Multiple strong narrow pulses can be used to emulate the effects of a single weak long pulse. (ref 1 figure 4.23)
When using monophasic pulses, it is very important to reverse the polarity regularly to avoid negative electrochemical effects.
Muscle vs sensory nerve stimulation
Literature says $\tau$ for nerve stimulation is around 200 to 300 $\mu s$. For muscle stimulation $\tau$ is around 2000 to 3000 $\mu s$. We can use this to bias our stimulation signal towards either nerves or muscle cells.
Suppose that we send 10 weak monophasic pulses with an interval of $500\mu s$. The time constant for nerve cells is short, total nerve response will be similar to a single weak pulse. The time constant for muscle cells is long, total muscle response will be about 10x larger than for a single pulse. This stimulation strategy can be used to bias stimulation towards muscle activation. Onwrikbaar tested that this does indeed result in increased muscle stimulation.
This does not work with biphasic pulses. If your device can only handle biphasic pulses, low frequencies are marginally more suited for muscle stimulation than high frequencies.
Pulse shape vs activation threshold
Literature says the difference between biphasic sine and square waves is very small at clinically relevant pulse widths. (ref 1, figure 4.16).
For short biphasic pulses, there is a difference in activation threshold for anode-first and cathode-first pulses. This effect disappears for pulse widths larger than $\tau$.
Monophasic pulses are slightly more efficient than biphasic pulses. There also is about a factor 5 difference in activation threshold between anode-first and cathode-first waveforms. In clinical settings sometimes cathodic pulses are used for primary stimulus and anodic pulses for electrochemical reasons.
The orientation of nerves in the skin is random, so it is difficult to control whether nerves are stimulated anode-first or cathode-first in typical stim setups.
cathodic pulses followed by anodic pulses can sometimes infer with action-potential generation, it is recommended to have $100\mu s$ between phases to avoid this effect and improve energy efficiency.
Pulse shape vs depth
Skin resistance is highly nonlinear, the different layers of the skin have varying properties that depend on the frequency. Adjusting the pulse shape (adding or removing high-frequency components) therefore can have impact on the depth of stimulation.
This is illustrated below:
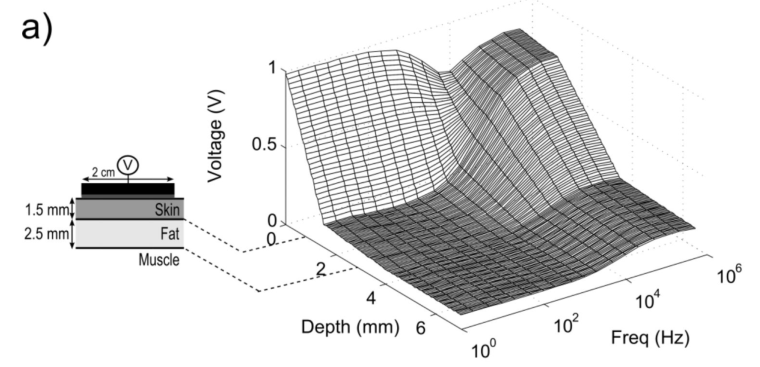
(Volume conductor model of transcutaneous electrical stimulation with kilohertz signals)
Based on this source we can conclude:
- With low frequency signals, the voltage rapidly decreases as it passes through the skin. The nerves close to the surface receive lots of stimulation, deeper nerves receive less. Not a lot of voltage makes it to the fat mass / muscles.
- As the frequency increases, the voltage curve over the skin flattens out, nerve ending in the skin see the same voltage regardless of depth. More signal makes it though the skin to the fat mass and muscles.
In summary, high frequency signals are useful for:
- Stimulating deeper nerves
- Stimulating muscles (?)
Low frequency signals are useful for:
- Stimulating nerves close to the surface
Not much is known about practical implications.
Selective activation
TODO: research
Using conventional electrical stimulation waveforms with relatively narrow pulses, the largest diameter fibers are activated at the lowest stimulus amplitude. In motor nerves, activating large diameter fibers first corresponds to activating the largest motor units first. This recruitment order is opposite of the physiological case where the smallest motor units are recruited first.
From Electrical stimulation of excitable tissue: design of efficacious and safe protocols
This paper also looks useful: Electrical stimulation of mechanoreceptors https://iopscience.iop.org/article/10.1088/1742-6596/332/1/012044/pdf
Blocking effects
Specific waveforms can exhaust the nerves ability to fire, which may be useful for highly targeted sensations. However, this technique seems impractical for usage in typical stim setups.
Reversible Nerve Conduction Block Using Kilohertz Frequency Alternating Current
References
- Applied Bioelectricity - From Electrical Stimulation to Electropathology, Chapter 4.