Research - Technology-for-the-Poorest-Billion/2024-LiFETIME GitHub Wiki
Project Brief and Connections with Literature
- Wide range of degradation modes -> limits ability to make use of capacity.
- Reuse or recycle??? See the stuff I saw on LinkedIn
- Possible approaches:
- Intrusive techniques - More and better data, but typically destroys battery.
- Reconstruction with new electrodes. -> See "Performance of LiNiCoO2 materials for advanced lithium-ion batteries|(2)"
- Analysis of gas generation -> See "Gas evolution behaviors for several cathode materials in lithium-ion batteries.|(3)"
- Calorimetry -> See "Thermal stability of LiPF6/EC + DEC electrolyte with charged electrodes for lithium ion batteries.|(4)"
- Non-Intrusive - Allows continued operation and in-situ monitoring but costly (in time and/or money)
- Cell Cycling
- Single 2.5Ah cell takes 2.5hrs to charge @1A. Slow charging provides better insight and is less harsh -> see "Rate dependency of incremental capacity analysis (dQ/dV) as a diagnostic tool for lithium-ion batteries.|(7)". Results in tradeoff. -> unsuitable for rapid or in-situ testing.
- Electrochemical Impedance Spectroscopy (EIS) -> See "Electrochemical impedance spectroscopy for lithium-ion cells Test equipment and procedures for aging and fast characterization in time and frequency domain.|(5)"
- Incremental Capacity Analysis -> See "Best practices for incremental capacity analysis.|(6)"
- Cell Cycling
- "Middle Ground" -> Physically informed modelling
- "Digital twin" of the cell -> can predict key factors (capacity, likely failure modes).
- Equivalent Circuit Modelling.
- eg. represent dielectric as a capacitor with leakage resistance.
- Can use varying degrees of complexity in the model.
- Intrusive techniques - More and better data, but typically destroys battery.
The goal is to develop a method of NDT that can be used to obtain model parameters. NDT limits parameters to measurable or inferable characteristics. We have a small dataset of cycling and EIS measurements of 18650 cells.
High-Level / Introductory Reading
Most of this comes from Wikipedia and from reading the sources cited in the page, but the results of them can be summarised as:
Lithium ion batteries work by adding and removing lithium ions into a solid, conducting solid's structure. Negative (anode when discharging) electrode typically graphite. Positive (cathode when discharging) is a metal oxide or phosphate. Electrolyte (conducts ions not electrons) is a lithium salt (ionic compound) in an organic solvent. Separator prevents the positive and negative layers from shorting. The Lithium ions move between the two electrode materials via the conducting electrolyte solution.
Factors affecting cycle life:
- Temperature (strongly dependent. low degradation 5<T<35)
- Current (Charge/Discharge)
- Depth of discharge
Some researchers use cumulative discharge as a measure of battery age.
Degradation Mechanisms
From: Vermeer, W.; Mouli, G. R. C.; Bauer, P. - IEEE TRANSACTIONS ON TRANSPORTATION ELECTRIFICATION, VOL. 8, NO. 2, JUNE 2022\10.1109/TTE.2021.313835

- Reduction of organic carbonate electrolyte at anode -> Solid Electrolyte Interface grows -> Irreversibly traps $Li+$ , reducing amount available for battery storage.
- Indicators -> Ohmic Impedance increases, reduced charge.
- Antecedents -> At const T, interface film thickness $\propto$ time spent in charged state. Doesn't occur with titanate anodes vs graphite nodes.
- Risks -> At high T, or under damage the electrolyte reduction can proceed explosively.
- Lithium metal plating -> reduces Li+ availability + causes internal short circuiting and combustion.
- Indicators -> Larger slopes of capacity loss and resistance increase per cycle.
- Antecedents -> Fast charging, low temperatures increase likelihood.
- Risks -> Combustion.
- Loss of (+ or -, eg Mn3+) electroactive materials due to dissolution cracking, exfoliation, detachment or volume change during cycling causes strains.
- Indicators -> Increased resistance (lower power), charge reduces.
- Structural degradation of cathode materials such as Li+, Ni2+ cation mixing with nickel-rich materials.
- Indicators -> Electrode saturation, loss of charge, "voltage fade" -> #todo what does this mean
- Negative copper current collector corrosion/dissolution
- Antecedents -> Low cell voltages
- PVDF binder degradation -> causes detachment of electroactive materials, loss of Ah charge
Theories from researches? Anode aging responsible for most of the capacity loss. (1) Manganese cathodes age faster possibly due to the Mn ion dissolution (3) Theorise that degradation follows same pathways at 25 as 50C but with half speed.
Paper Summaries
Analysis of Lithium-Ion Battery Models Based on Electrochemical Impedance Spectroscopy.
Westerhoff, U., Kurbach, K., Lienesch, F. & Kurrat, M. Analysis of Lithium-Ion Battery Models Based on Electrochemical Impedance Spectroscopy. Energy Technol. 4, 1620–1630 (2016).
What does the paper do?
- Presents and evaluates equivalent circuit models based on accuracy (based on difference between simulated and measured impedance spectra) and parameterization time.
- Impedance spectra measured at different states of
- Charge
- Health
- Temperature
- Parameters extracted using least-squares and the[ Levenberg-Marquardt](Levenberg–Marquardt algorithm - Wikipedia) algorithm.
Introduction
- Basic structure of battery has to be known.
- Can use an "each layer in series" approach, where each layer of the cell is represented by an equivalent circuit.
- OR use the density function of the distribution of relaxation times (DRT) to determine the optimal equivalent circuit configuration (Number of RC elements required).
- Simplified version: Find minima, maxima and inflection points in the Nyquist plot.
Modelling
- You only need resistors, inductors, capacitors to represent the behaviour.
- Most battery systems can already be described with sufficient accuracy using these models #todo read 13-15. what is meant by battery system.
- If you want to add dependencies for (below) -> Use "Constant Phase Elements"
- State of charge
- Temperature
- State of health
Constant-Phase Elements
- Accounts for real electrode plate behaviour. Diffusion is highly dependent on particle size distribution.
- Diffusion is a slow process -> Warburg element used.
- For diffusion: Impedance spectrum Nyquist extends with 45 degree slope.
Parameter Estimation
- #todo read.
EIS
-
20kHz measurement setup has significant inductive effect.
- 1k-10mHz sees:
- Charge-transport process in the electrolyte, SEI interphase, and active material (incl anode, cathode)
Results
- 1 RC element sufficient for lower dynamics. Charge-transfer resistance, state of charge and state of health.
- 2 elements can represent the range of diffusion processes.
- 3 allows accurate impedance spectrum analysis.
- 5 RC or a constant phase element promising for larger dynamics or diagnostics. This also requires significantly more iterations to determine the parameters. 1RCPE is better as yields similar results for far fewer iterations. #todo read the rest
Conclusions
#todo read the rest
Best practices for incremental capacity analysis.
Dubarry, M. & Anseán, D. Best practices for incremental capacity analysis. Front. Energy Res. 10, (2022) There is a 2023 correction to this article, where they had accidentally placed figure 12 twice.
- Incremental capacity analysis $\frac{dQ}{dV} = f(V)$, first introduced by #Bloom-2005 can identify battery degradation modes.
- Current, voltage (things we already sense) => material chemistry/thermo => state of battery.
- Analysis is chemistry dependent -> unknown generality -> Literature is a "minefield" according to [#Xu-2022]
- Article provides synthetic and real training set. #todo unfinished
Other Papers from LiFETIME
Performance of LiNiCoO2 materials for advanced lithium-ion batteries:
Itou, Y. & Ukyo, Y. Performance of LiNiCoO2 materials for advanced lithium-ion batteries. J. Power Sources 146, 39–44 (2005)
Gas evolution behaviors for several cathode materials in lithium-ion batteries.
Kong, W., Li, H., Huang, X. & Chen, L. Gas evolution behaviors for several cathode materials in lithium-ion batteries. J. Power Sources 142, 285–291 (2005).
Thermal stability of LiPF6/EC + DEC electrolyte with charged electrodes for lithium ion batteries.
Wang, Q., Sun, J., Yao, X. & Chen, C. Thermal stability of LiPF6/EC + DEC electrolyte with charged electrodes for lithium ion batteries. Thermochim. Acta 437, 12–16 (2005).
Electrochemical impedance spectroscopy for lithium-ion cells: Test equipment and procedures for aging and fast characterization in time and frequency domain.
Lohmann, N., Weßkamp, P., Haußmann, P., Melbert, J. & Musch, T. Electrochemical impedance spectroscopy for lithium-ion cells: Test equipment and procedures for aging and fast characterization in time and frequency domain. J. Power Sources 273, 613–623 (2015).
Best practices for incremental capacity analysis.
Dubarry, M. & Anseán, D. Best practices for incremental capacity analysis. Front. Energy Res. 10, (2022) There is a 2023 correction to this article, where they had accidentally placed figure 12 twice.
- Incremental capacity analysis $\frac{dQ}{dV} = f(V)$, first introduced by #^Bloom-2005 can identify battery degradation modes.
- Current, voltage (things we already sense) => material chemistry/thermo => state of battery.
- Analysis is chemistry dependent -> unknown generality -> Literature is a "minefield" according to #^Xu-2022
- Article provides synthetic and real training set. #todo unfinished
Rate dependency of incremental capacity analysis (dQ/dV) as a diagnostic tool for lithium-ion batteries.
Fly, A. & Chen, R. Rate dependency of incremental capacity analysis (dQ/dV) as a diagnostic tool for lithium-ion batteries. J. Energy Storage 29, 101329 (2020).
Further Reading
More on ICA
Xu-2022
Xu, K. (2022). Navigating the minefield of battery literature. Commun. Mat. 3(1), 31. doi:10.1038/s43246-022-00251-5
Incremental capacity analysis / Differential Voltage
Bloom-2005
Bloom, I., Jansen, A. N., Abraham, D. P., Knuth, J., Jones, S. A., Battaglia, V. S., et al. (2005). Differential voltage analyses of high-power, lithium-ion cells: 1. Technique and application. J. Power Sources 139 (1–2), 295–303. doi:10.1016/j. jpowsour.2004.07.021
Electrochemical Impedance Spectroscopy
- Detects the electrochemical reaction happening in the battery
- Apply an electric signal with various frequencies to the battery system
- Output is a spectrum with a real and imaginary part
- Captures change in resistance of the battery
- EIS spectrum is a nyquist plot as a function of frequency
- It is non-invasive to obtain spectrum, i.e. no damage to battery
- Quick, 10-15 minutes, real-time
EIS spectrum changes with cycle number, making it a good indicator of degradation and SOH (State of Health)
EIS data is interpreted using an ECM, using a parametric model Different ECM for each battery system
Key degradation modes of Lithium-ion batteries:
LLI: Loss of lithium inventory LAM: Loss of active materials ORI: Ohmic resistance increase FRD: Faradic Rate Degradation
SEI layer formation
- Permanently binds some Li
- Reduces capacity as less Li+ available to move
Lithium plating
- Occurs when charging at cold temperatures or fast charging
- Graphite can’t accept the Li+ well or quickly enough so Li builds up as a plate
- This can lead to dendritic formation which can even stretch across the electrolyte and through the separator to cause short circuiting
Particle fracture
- Caused by thermal cycling
- Opens up more surface area for SEI to occur leading to more capacity loss
Structural disordering
- Happens in the presence of moisture
- Electrolyte reacts to form acidic HF
- This acid then reacts with the lithium oxide at the cathode, causing loss of electrolyte and reducing Li availability
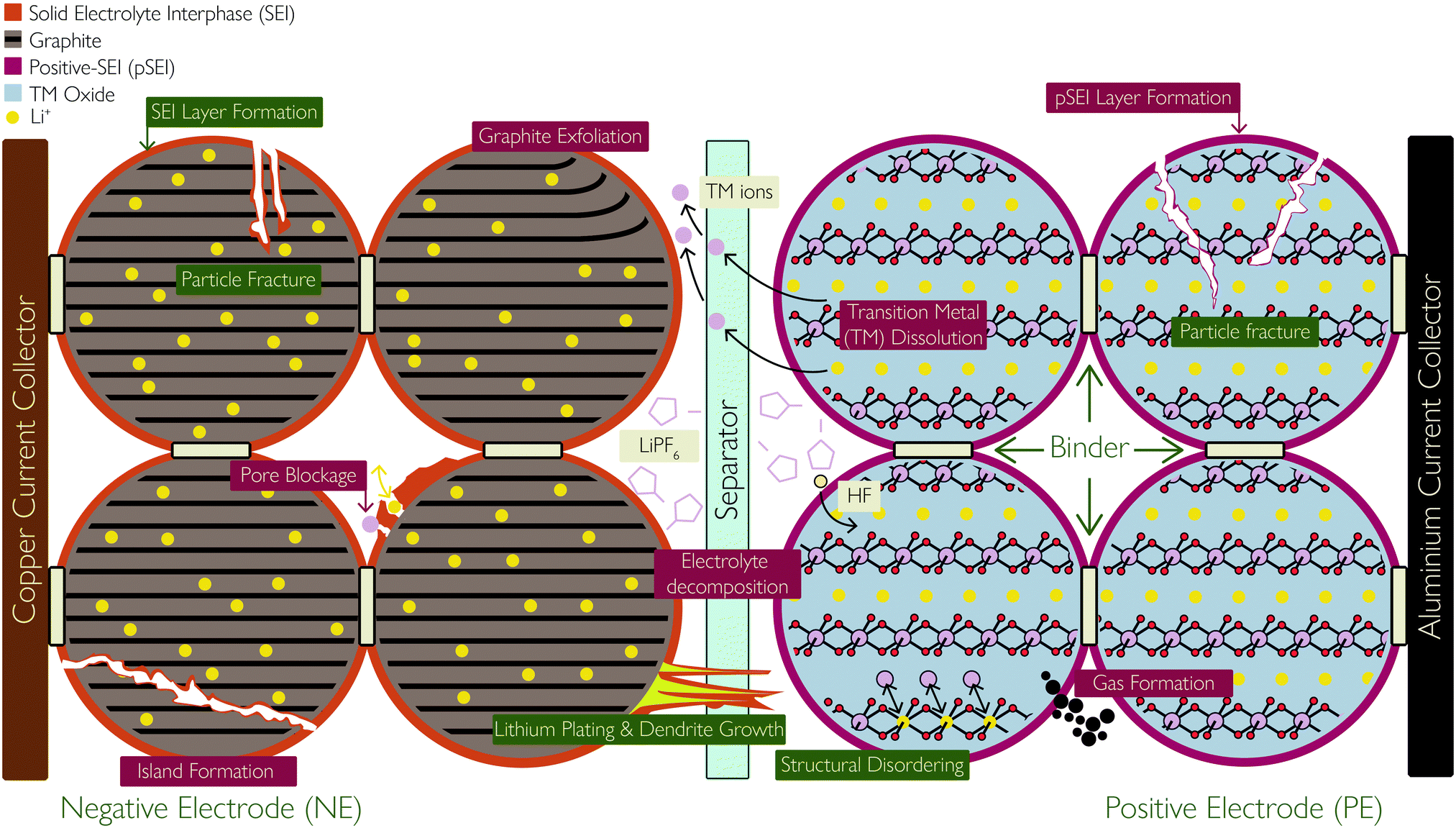
Reference: Edge, Jacqueline & O'Kane, Simon & Prosser, Ryan & Kirkaldy, Niall & Patel, Anisha & Hales, Alastair & Ghosh, Abir & Ai, Weilong & Chen, Jingyi & Jiang, Jason & Li, Shen & Pang, Mei-Chin & Bravo Diaz, Laura & Tomaszewska, Anna & Marzook, Mohamed & Radhakrishnan, Karthik & Wang, Huizhi & Patel, Yatish & Wu, Billy & Offer, Gregory. (2021). Lithium Ion Battery Degradation: What you need to know. Physical Chemistry Chemical Physics. 23. 10.1039/D1CP00359C.
Predicting the "Knee" in battery degradation
https://iopscience.iop.org/article/10.1149/1945-7111/ac6d13
- "Knee" is the point where rapid capacity degradation begins.
- Some mechanisms are easily detected via electrochemical measurement (3/6)
- The other 3 are more difficult, more work needed.
- Knee => End of Life. Reverse is not true, as cell can reach 80% whilst still having plenty of operational life left (ie not having reached the knee).
- ML should be able to predict the first 3, other methods have been reasonably successful at predicting the knee.
- Further reading -> What does the post-knee behaviour look like and is the cell still usable post-knee? If it isn't usable post-knee then knee prediction can be used as a definition of end-of-life. -> Read https://www.sciencedirect.com/science/article/pii/S2666546820300069 once post-knee behaviour known. Could be useful in informing ML approaches to knee prediction.
Some initial thoughts on applying ML to this problem:
Microsoft have done it!
- Report goes through A LOT of methods and how they performed
- Data from ~30 lithium ion batteries being charged and discharged until degradation from an experiment
- We need a variable for degradation
- State of Health (SOH): current capacity/ original capacity
- We need some understanding of how different failure modes change the readings and the rate of degradation
- Can apply ML to unsupervised learning for a broad approach or use inference to obtain ECM parameters (more likely option)
Objective: We want to be able to take a battery that has been used and tell how much it has degraded from a small sample of charging data from that battery.
A battery is a nonlinear, time-invariant system, so we need a non-linear approach such as GPs, NNs, GBM etc.
An interesting paper that used GPs:
Zhang, Y., Tang, Q., Zhang, Y. et al. Identifying degradation patterns of lithium ion batteries from impedance spectroscopy using machine learning. Nat Commun 11, 1706 (2020). https://doi.org/10.1038/s41467-020-15235-7
A talk and GitHub link for this group: https://youtu.be/_lkkm3CHuJA?si=D884nAByqnKl3JT3 https://github.com/rochan17/degradation-patterns-of-lithium-ion-batteries-from-impedance-spectroscopy
ECM fitting for EIS
Zahner Analysis: https://www.youtube.com/watch?v=407s6ySDBZk
PSTrace: https://www.youtube.com/watch?v=KQzp3lPMSxg
How different components contribute to the impedance spectrum: From 15 minutes https://www.youtube.com/watch?v=kexDd0kFAK8
Microsoft BatteryML
3.2 Feature Engineering
This section of the paper outlines useful degradation features.
Within-cycle features:
- QdLinear is a linear interpolation of the capacity-voltage curve in discharge cycles.
- Coulombic Efficiency is how efficiently a battery releases its stored energy. (Charge/Discharge capacity)
- Internal Resistance
Bewteen-cycle features:
(copied from the paper)
- Variance of the difference of QdLinear curves. (Severson et Al 2019) outlines how this indicates battery degradation speed. - comment: This is useful for training, but how practical is this metric for testing as we can't capture it in a single cycle.
- Capacity decay dynamics is the slope of capacity decay curve fitted in early cycles.
- Average charging time, which reflects the irreversible structural changes within the battery, such as lithium plating and the growth of the SEI layer.
- Temperature dynamics, which indicates the intensity of the electrochemical reactions occurring within the battery.
- Minimal internal resistance, reflecting the upper bound of battery health state
C.1 Remaining Useful Life Prediction
- Table 2
- Dummy Regressor - Baseline using the mean of the training label for prediction.
- Variance, Discharge and Full models - Linear regression based on hand-selected features.
- Variance model ony considers the variance of the QdLinear curve. (Linear interpolation of the capacity-voltage curve vs cycle count.)
C.2 State of Health Estimation
- Reference performance test used to standardise test conditions.
- There is a disparity between these test conditions and real-world workloads.
- They define SOH as the ratio of the battery's maximum discharge to nominal capacity per cycle.
- As degradation progresses variances in charging distribution increase.
- Tree-based models perform great across many datasets.
- Deep learning yet to surpass traditional methods for SOH estimation.
- There's a contradiction in current battery research. SOH has to be based off of standardised workloads, yet is applied to wildly varying real-world cases.
C.3 State of Charge Estimation
- SOC = SOC(SOH, discharge capacity)
- In their tests, they use a realistic workload for SOC.
- They extract current, voltage, time, charge and discharge capacity curves from preceding cycles.
- Tree models also perform best in this task.
- Linear still performs better than ML.
D Ablation Study
Ablation is the process of removing components from an AI system or model to understand what that component contributes.
The Knees Paper
One of the best papers I've read on the topic, and the source of my information for the project proposal presentation. Explains why people see sudden capacity loss in phone batteries etc.
Review - "Knees" in Lithium-Ion Battery Aging Trajectories, Peter M. Attia et al 2022 J. Electrochem. Soc. 169 06051 https://iopscience.iop.org/article/10.1149/1945-7111/ac6d13/pdf
How do EIS characteristics relate to cell characteristics?
Biologic.net Why use EIS for battery research? (June 2023) https://www.biologic.net/topics/why-use-electrochemical-impedance-spectroscopy-for-battery-research/
 Doesn't cite a source, but information and image is also in this presentation https://fbicrc.com.au/wp-content/uploads/2020/03/Session-2-FBICRC-participant-summit-presentations.pdf
Doesn't cite a source, but information and image is also in this presentation https://fbicrc.com.au/wp-content/uploads/2020/03/Session-2-FBICRC-participant-summit-presentations.pdf
See if we can find info replicating this elsewhere. This article suggests that high frequencies correspond to the ohmic resistance of the electrolyte, the middle frequencies to SEI capacitance and the electron transfer rate and the low frequencies give information about the diffusion processes of species within the insertion material at the negative electrode.