Wound Healing Tool - MontpellierRessourcesImagerie/imagej_macros_and_scripts GitHub Wiki
The MRI Wound Healing Tool can be used to analyze scratch assays. It measures the area of a wound in a cellular tissue on a stack of images representing a time-series. An example image can be found here. An example image for the find-edges method (see below) can be found here.
Getting Started
To install the tools, save the file MRI_Wound_Healing_Tool.ijm under macros/toolsets in your ImageJ installation.
Select the MRI Wound Healing Tool toolset from the >> button of the ImageJ launcher.

- the first button opens this help-page.
- the m button starts the measurement on the active stack
- the b button measures all tif-stacks in a folder
Options
By right-clicking on the m button you can open the options dialog.

- method: You can choose between a variance based method and a method based on find edges. Note that for find edges the parameters variance-filter-radius and threshold are not taken into account. Use for example the parameters 20, 1, 4 and 999999 on the example image for the variance case.
- variance filter radius: The radius of the variance filter that is applied to separate the zone occupied by tissue from the empty zone. The radius must be big enough so that the variance due to the tissue plays a role compared to the variance of the noise in the image. Note that the calculation time becomes longer with a bigger radius.
- threshold The image resulting from the variance filter is converted to a mask by applying the given threshold. If the input images are 16bit the threshold 1 will probably work.
- radius open This operation will close holes in the tissue. It must be big enough to close small holes in the tissue and small enough to not close the area of the wound in later images.
- min. size Minimum size from which on the wound is taken into account. This excludes small remaining holes in the tissue.
- ignore spatial calibration If checked the measurements will be in pixel otherwise the spatial calibration of the image if any is used.
Hints
- If you have the frames of a timeseries of a closing scratch as individual image-files, open them as a stack in FIJI/ImageJ, before running the analysis. This way you will get all measurements in one table, instead of having one table per image. You can use the command
File>Import>Image Sequenceto open a series of images as a stack. - In ImageJ, select Analyze>Set Measurements to select the features you want to measure. You should at least select area and Display label.
- It can happen that the result at one time-point consists of more then one area. You can recognize it because they have the same number in the column slice of the results table. To get the total area for one time-point you need to add all the surfaces measured for that time-point
Results

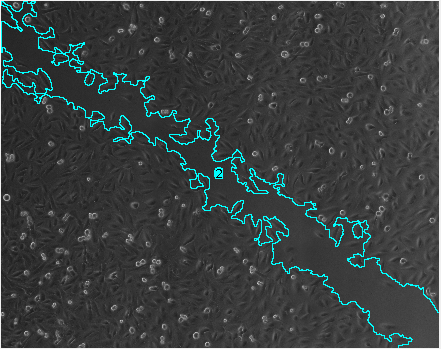
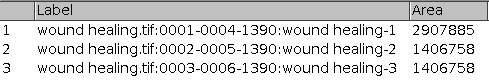
How to cite the tool
You can use the tool's resource id (see scicrunch.org):
(Wound Healing Tool, RRID:SCR_025260)
History
- 09.05.2022 Refactored and fixed the border problem. The tool should now be independent from ImageJ's global settings and the gap now goes to the border of the image instead of stopping at a distance depending on the number of iterations of the morphological
close-operation.
See also
Other (third party) software for scratch assay analysis
- CSMA_WoundHealing - Uses opencv and skimage, but runs from ImageJ
- Wound healing - a CellProfiler pipeline
- Wound-healing-size-tool, another ImageJ macro-tool
- ScratchAssayAnalyzer in MiToBo - A microscope image analysis toolbox
- Robust quantitative scratch assay runs in Matlab or Octave
- TScratch, Matlab with Windows and Mac binaries
- Hight-Throughput Microscopy Wound healing tool, Matlab
- PyScratch, Python, the github repository is here.
Videos
Publications using this tool
-
Thanh Pham, T., Sagymbayeva, A., Elebessov, T., Onzhanova, Z., Molnár, F., 2025. CSMA: A Standalone and ImageJ-Compatible Tool for Enhanced Wound Healing Assay Analysis. IEEE Access 13, 69341–69352.
-
Pessa, J.C., Paavolainen, O., Hästbacka, H.S.E., Puustinen, M.C., Da Silva, A.J., Pihlström, S., Gramolelli, S., Boström, P., Hartiala, P., Peuhu, E., Joutsen, J., Sistonen, L., 2025. HSF2 drives breast cancer progression by acting as a stage-specific switch between proliferation and invasion. Sci. Adv. 11, eady1289.
-
Jones, L., Divakar, S., Collins, L., Hamarneh, W., Ameerally, P., Anthony, K., Machado, L., 2025. Duchenne muscular dystrophy gene product expression is associated with survival in head and neck squamous cell carcinoma. Sci Rep 15, 10754.
-
Yamaguchi, H., González-Duarte, R.J., Qin, X.-Y., Abe, Y., Takada, I., Charroy, B., Cázares-Ordoñez, V., Uno, S., Makishima, M., Esumi, M., 2025. Transglutaminase 2 Stimulates Cell Proliferation and Modulates Transforming Growth Factor-Beta Signaling Pathway Independently of Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma Cells. Int J Mol Sci 26, 5497.
-
Nacka-Aleksić, M., Pirković, A., Vilotić, A., Kosanović, M., Dekanski, D., Legner, J., Jovanović Krivokuća, M., 2025. Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence. Life (Basel) 15, 918.
-
Pandey, P., Arya, D.K., Kumar, A., Kaushik, A., Mishra, Y.K., Rajinikanth, P.S., 2025. Dual ligand functionalized pH-sensitive liposomes for metastatic breast cancer treatment: in vitro and in vivo assessment. J. Mater. Chem. B 13, 2682–2694.
-
Syama, H.P., Unnikrishnan, B.S., Sreekutty, J., Archana, M.G., Preethi, G.U., Reshma, P.L., Sreelekha, T.T., 2025. Polysaccharide-capped silver nanoparticles impregnated cream for the efficient management of wound healing. RSC Pharm. 10.1039.D4PM00274A.
-
Krishnamoorthy, V.K., Hamdani, F., Shukla, P., Rao, R.A., Anaitullah, S., Biligiri, K.K., Kadumuri, R.V., Pothula, P.R., Chavali, S., Rampalli, S., 2025. NSD3 protein methylation and stabilization transforms human ES cells into variant state. Life Sci. Alliance 8, e202402871.
-
Santhosh, S.K., Sarojini, S., 2025. Antibiofilm and anti-quorum properties of ethanolic leaf extracts of Syzygium jambos and Psidium guajava and their gel formulation for wound healing applications. Plant Sci. Today.
-
Lin, Y., Silverman-Dultz, A., Bailey, M., Cohen, D.J., 2024. A programmable, open-source robot that scratches cultured tissues to investigate cell migration, healing, and tissue sculpting. Cell Reports Methods 4, 100915.
-
Pessa, J.C., Paavolainen, O., Puustinen, M.C., Hästbacka, H.S.E., Da Silva, A.J., Pihlström, S., Gramolelli, S., Boström, P., Hartiala, P., Peuhu, E., Joutsen, J., Sistonen, L., 2024. Dynamic HSF2 regulation drives breast cancer progression by steering the balance between proliferation and invasion.
-
Park, J.Y., Kim, H.-S., Hyung, H., Jang, Soyeon, Ko, J., Lee, J.H., Kim, S.-Y., Park, Song, Yi, J., Park, Sijun, Lim, S.-G., Kim, S., Lee, S., Kim, M.O., Jang, Soyoung, Ryoo, Z.Y., 2024. TASL mediates keratinocyte differentiation by regulating intracellular calcium levels and lysosomal function. Sci Rep 14, 10978.
-
Jiang, J., Lin, Chi‐Hung, Chang, T., Lo, L., Lin, Chien‐Ping, Lu, R., Yang, C., 2024. Decreased interleukin‐ 17RA expression is associated with good prognosis in patients with colorectal cancer and inhibits tumor growth and vascularity in mice. Cancer Medicine 13, e7059.
-
Lovatt, C., Williams, M., Gibbs, A., Mukhtar, A., Morgan, H.J., Lanfredini, S., Olivero, C., Spurlock, G., Davies, S., Philpott, C., Tovell, H., Turnpenny, P., Baban, D., Knight, S., Brems, H., Sampson, J.R., Legius, E., Upadhyaya, M., Patel, G.K., 2024. Pigment Epithelium Derived Factor Drives Melanocyte Proliferation and Migration in Neurofibromatosis Café Au Lait Macules. Skin Health and Disease 4, ski2.394.
-
Jiang, J., Lin, Chi‐Hung, Chang, T., Lo, L., Lin, Chien‐Ping, Lu, R., Yang, C., 2024. Decreased interleukin‐ 17RA expression is associated with good prognosis in patients with colorectal cancer and inhibits tumor growth and vascularity in mice. Cancer Medicine 13, e7059. 10.1002/cam4.7059
-
Enxian, Shi, 2024, Functional characterization of effectors of EGFR-mediated local invasion in head and neck carcinomas. Universität München
-
Rossini, S., Ambrosino, S., Volpi, C., Belladonna, M.L., Pallotta, M.T., Panfili, E., Suvieri, C., Macchiarulo, A., Mondanelli, G., Orabona, C., 2024. Epacadostat stabilizes the apo-form of IDO1 and signals a pro-tumorigenic pathway in human ovarian cancer cells. Front. Immunol. 15, 1346686. 10.3389/fimmu.2024.1346686
-
Acebes-Huerta, A., Martínez-Botía, P., Carbajo-Argüelles, G., Fernández-Fuertes, J., Muñoz-Turrillas, M.C., Ojea-Pérez, A.M., López-Vázquez, A., Eble, J.A., Gutiérrez, L., 2024. Characterization of the molecular composition and in vitro regenerative capacity of platelet-based bioproducts and related subfractions. Acta Biomaterialia 177, 132–147. 10.1016/j.actbio.2024.01.029
-
Cano, I., Wild, M., Gupta, U., Chaudhary, S., Ng, Y.S.E., Saint-Geniez, M., D’Amore, P.A., Hu, Z., 2024. Endomucin selectively regulates vascular endothelial growth factor receptor-2 endocytosis through its interaction with AP2. Cell Commun Signal 22, 225.
-
Liedtke, V., 2024. Lens epithelium-derived growth factor (LEDGF) as an aid in the diagnosis of benign prostatic hyperplasia (BPH) and the molecular principles of LEDGF in human cancer cells. BTU Cottbus - Senftenberg.
-
Tran, S., Sipila, P., Thakur, S., Zhang, C., Narendran, A., 2024. Identification and In Vivo Validation of Unique Anti-Oncogenic Mechanisms Involving Protein Kinase Signaling and Autophagy Mediated by the Investigational Agent PV-10. Cancers 16, 1520. 10.3390/cancers16081520
-
Parrella, P., Barbano, R., Jonas, K., Fontana, A., Barile, S., Rendina, M., Lo Mele, A., Prencipe, G., Ciuffreda, L., Morritti, M.G., Valori, V.M., Graziano, P., Maiello, E., Copetti, M., Pichler, M., Pasculli, B., 2024. Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer. Biomedicines 12, 2625.
-
Kukulage, Dhanushika S.K. et al. 2024, Protein phosphatase PP2Cα S-glutathionylation regulates cell migration. Journal of Biological Chemistry, Volume 300, Issue 10, 107784
-
Crnkovic, S., Puthenparampil, H.T., Mulch, S., Biasin, V., Wilhelm, J., Bartkuhn, M., Rad, E.B., Wawrzen, A., Matzer, I., Mitra, A., Leib, R., Nagy, B.M., Sahu-Osen, A., Valzano, F., Bordag, N., Evermann, M., Hoetzenecker, K., Olschewski, A., Ljubojevic-Holzer, S., Wygrecka, M., Stenmark, K., Marsh, L.M., De Jesus Perez, V., Kwapiszewska, G., 2024. Adventitial fibroblasts direct smooth muscle cell-state transition in pulmonary vascular disease.
-
Akinlalu, A., Flaten, Z., Rasuleva, K., Mia, M.S., Bauer, A., Elamurugan, S., Ejjigu, N., Maity, S., Arshad, A., Wu, M., Xia, W., Fan, J., Guo, A., Mathew, S., Sun, D., 2024. Integrated proteomic profiling identifies amino acids selectively cytotoxic to pancreatic cancer cells. The Innovation 5, 100626. 10.1016/j.xinn.2024.100626
-
Crespo, B., Illera, J.C., Silvan, G., Lopez-Plaza, P., Herrera De La Muela, M., De La Puente Yague, M., Diaz Del Arco, C., De Andrés, P.J., Illera, M.J., Caceres, S., 2024. Bicalutamide Enhances Conventional Chemotherapy in In Vitro and In Vivo Assays Using Human and Canine Inflammatory Mammary Cancer Cell Lines. IJMS 25, 7923.
-
Cresens, C., Montero-Calle, A., Solís-Fernández, G., Shaghaghi, B., Gerrits, L., Aytekin, S., Kouwer, P.H.J., Barderas, R., Rocha, S., 2024. Deciphering stiffness-driven changes in colorectal cancer by proteomics.
-
Abdallah, S., Abu-Reidah, I., Mousa, A., 2024. Phytochemical Components, Hindering Abilities on Cell Proliferation, Cell Migration and Three-Dimensional Spheroids’ Formation Capacity of Micromeria fruticosa Infusion. Natural Product Communications 19, 1934578X241275363. 10.1177/1934578X241275363
-
Mojahedi, M., Zargar Kharazi, A., and Poorazizi, E. (2024). Preparation and characterization of carboxymethyl cellulose/polyethylene glycol films containing bromelain/curcumin: In vitro evaluation of wound healing activity. Polymer Engineering & Sci 64, 1993–2005.
-
Kesidou, D., Bennett, M., Monteiro, J.P., McCracken, I.R., Klimi, E., Rodor, J., Condie, A., Cowan, S., Caporali, A., Wit, J.B.M., et al. (2024). Extracellular vesicles from differentiated stem cells contain novel proangiogenic miRNAs and induce angiogenic responses at low doses. .Molecular Therapy 32, 185–203.
-
Rojas, L., Tobar, N., Espinoza, J., Ríos, S., Martínez, C., Martínez, J., Graves, D.T., and Smith, P.C. (2024). FOXO1 regulates wound‐healing responses in human gingival fibroblasts. J of Periodontal Research 59, 611–621.
-
Waters, E., Pucci, P., Rahman, R., Yatsyshyna, A.P., Porter, H., Hirst, M., Gromnicova, R., Kraev, I., Mongiardini, V., Grimaldi, B., Golding, J., Fillmore, H.L., Győrffy, B., Gangadharannambiar, P., Velanis, C.N., Heath, C.J., Crea, F., 2024. REST-dependent glioma progression occurs independently of the repression of the long non-coding RNA HAR1A. PLoS ONE 19, e0312237.
-
Heydari, P., Zargar Kharazi, A., and Shariati, L. (2024). Enhanced wound regeneration by PGS/PLA fiber dressing containing platelet-rich plasma: an in vitro study. Sci Rep 14, 12019.
-
Ruta, V., Naro, C., Pieraccioli, M., Leccese, A., Archibugi, L., Cesari, E., Panzeri, V., Allgöwer, C., Arcidiacono, P.G., Falconi, M., et al. (2024). An alternative splicing signature defines the basal-like phenotype and predicts worse clinical outcome in pancreatic cancer. Cell Reports Medicine 5, 101411. 10.1016/j.xcrm.2024.101411.
-
Serwe, G., Kachaner, D., Gagnon, J., Plutoni, C., Lajoie, D., Duramé, E., Sahmi, M., Garrido, D., Lefrançois, M., Arseneault, G., et al. (2023). CNK2 promotes cancer cell motility by mediating ARF6 activation downstream of AXL signalling. Nat Commun 14, 3560. 10.1038/s41467-023-39281-z.
-
Bellissima, A., Cucci, L.M., Sanfilippo, V., De Bonis, A., Fiorenza, R., Scirè, S., Marzo, T., Severi, M., La Mendola, D., Notarstefano, V., Giorgini, E., Satriano, C., 2023. Pd-Based Hybrid Nanoparticles As Multimodal Theranostic Nanomedicine. ACS Appl. Bio Mater. 6, 483–493.
-
Wongviriya, A., Shelton, R.M., Cooper, P.R., Milward, M.R., Landini, G., 2023. The relationship between sphingosine-1-phosphate receptor 2 and epidermal growth factor in migration and invasion of oral squamous cell carcinoma. Cancer Cell Int 23, 65. 10.1186/s12935-023-02906-w
-
Novohradsky, V., Marco, A., Markova, L., Cutillas, N., Ruiz, J., Brabec, V., 2023. Ir(III) Compounds Containing a Terdentate Ligand Are Potent Inhibitors of Proliferation and Effective Antimetastatic Agents in Aggressive Triple-Negative Breast Cancer Cells . J. Med. Chem. 66, 9766–9783. 10.1021/acs.jmedchem.3c00586
-
Um, H., Jeong, H., Lee, B., Kim, Y., Lee, J., Roh, J.S., Lee, S.-G., Park, H.R., Robinson, W.H., Sohn, D.H., 2023. FAT10 Induces cancer cell migration by stabilizing phosphorylated ABI3/NESH. Animal Cells and Systems 27, 53–60. 10.1080/19768354.2023.2186486
-
Gurri, S., Siegenthaler, B., Cangkrama, M., Restivo, G., Huber, M., Saliba, J., Dummer, R., Blank, V., Hohl, D., and Werner, S. (2023). NRF3 suppresses squamous carcinogenesis, involving the unfolded protein response regulator HSPA5. EMBO Mol Med 15, e17761. 10.15252/emmm.202317761.
-
Kukulage, D.S.K., Yapa Abeywardana, M., Matarage Don, N.N.J., Hu, R.-M., Shishikura, K., Matthews, M.L., Ahn, Y.-H., 2023. Chemoproteomic strategy identified p120-catenin glutathionylation regulates E-cadherin degradation and cell migration. Cell Chemical Biology 30, 1542-1556.e9.
-
Islam Khan, M.Z., Law, H.K.W., 2023. Suppression of small nucleolar RNA host gene 8 (SNHG8) inhibits the progression of colorectal cancer cells. Non-coding RNA Research 8, 224–232.
-
Rizzo, S., Sikorski, E., Park, S., Im, W., Vasquez‐Montes, V., Ladokhin, A.S., Thévenin, D., 2023. Promoting the activity of a receptor tyrosine phosphatase with a novel pH ‐responsive transmembrane agonist inhibits cancer‐associated phenotypes. Protein Science 32, e4742. 10.1002/pro.4742
-
Wu, C., Zhu, X., Dai, Q., Chu, Z., Yang, S., Dong, Z., 2023. SUMOylation of SMAD4 by PIAS1 in Conjunction with Vimentin Upregulation Promotes Migration Potential in Non-Small Cell Lung Cancer. Front. Biosci. (Landmark Ed) 28, 192. 10.31083/j.fbl2808192
-
Espinosa-Ruíz, C., and Esteban, M.Á. (2023). Modulation of cell migration and cell tracking of the gilthead seabream (Sparus aurata) SAF-1 cells by probiotics. Fish & Shellfish Immunology 142, 109149. 10.1016/j.fsi.2023.109149.
-
Huang, C.-J., Pu, C.-M., Su, S.-Y., Lo, S.-L., Lee, C.H., and Yen, Y.-H. (2023). Improvement of wound healing by capsaicin through suppression of the inflammatory response and amelioration of the repair process. Mol Med Rep 28, 155. 10.3892/mmr.2023.13042.
-
Jonas, K., Prinz, F., Ferracin, M., Krajina, K., Deutsch, A., Madl, T., Rinner, B., Slaby, O., Klec, C., Pichler, M., 2023. MiR-4646-5p Acts as a Tumor-Suppressive Factor in Triple Negative Breast Cancer and Targets the Cholesterol Transport Protein GRAMD1B. ncRNA 10, 2.
-
Kadunc Polajnar, L., Lainšček, D., Gašperšič, R., Sušjan-Leite, P., Kovačič, U., Butinar, M., Turk, B., Jerala, R., and Hafner-Bratkovič, I. (2023). Engineered combinatorial cell device for wound healing and bone regeneration. Front. Bioeng. Biotechnol. 11, 1168330. 10.3389/fbioe.2023.1168330.
-
Acosta, A.C., Joud, H., Sun, M., Avila, M.Y., Margo, C.E., and Espana, E.M. (2023). Keratocyte-Derived Myofibroblasts: Functional Differences With Their Fibroblast Precursors. Invest. Ophthalmol. Vis. Sci. 64, 9. 10.1167/iovs.64.13.9.
-
Schnoell, J., Sparr, C., Al-Gboore, S., Haas, M., Brkic, F.F., Kadletz-Wanke, L., Heiduschka, G., and Jank, B.J. (2023). The ATR inhibitor berzosertib acts as a radio- and chemosensitizer in head and neck squamous cell carcinoma cell lines. Invest New Drugs 41, 842–850. 10.1007/s10637-023-01408-w.
-
Papadaki, V., Erpapazoglou, Z., Kokkori, M., Rogalska, M.E., Potiri, M., Birladeanu, A., Tsakiri, E.N., Ashktorab, H., Smoot, D.T., Papanikolopoulou, K., et al. (2023). IQGAP1 mediates the communication between the nucleus and the mitochondria via NDUFS4 alternative splicing. NAR Cancer 5, zcad046. 10.1093/narcan/zcad046.
-
Gordon, E.R., Wright, C.A., James, M., and Cooper, S.J. (2023). Transcriptomic and functional analysis of ANGPTL4 overexpression in pancreatic cancer nominates targets that reverse chemoresistance. BMC Cancer 23, 524. 10.1186/s12885-023-11010-1.
-
Afshar, A., Khoradmehr, A., Nowzari, F., Baghban, N., Zare, M., Najafi, M., Keshavarzi, S.Z., Zendehboudi, F., Mohebbi, G., Barmak, A., Mohajer, F., Basouli, N., Keshtkar, M., Iraji, A., Sari Aslani, F., Irajie, C., Nabipour, I., Mahmudpour, M., Tanideh, N., Tamadon, A., 2023. Tissue Extract from Brittle Star Undergoing Arm Regeneration Promotes Wound Healing in Rat. Marine Drugs 21, 381. 10.3390/md21070381
-
Dardis, G.J., Wang, J., Simon, J.M., Wang, G.G., and Baldwin, A.S. (2023). An EZH2-NF-κB regulatory axis drives expression of pro-oncogenic gene signatures in triple negative breast cancer. iScience 26, 107115. 10.1016/j.isci.2023.107115.
-
Mayor, J., Cuesta, A., Espinosa-Ruíz, C., and Esteban, M.Á. (2023). In vitro effects of astaxanthin on bacterial and cell viability, cell migration and mitochondrial activities in four fish cell lines. Aquaculture Reports 31, 101636. 10.1016/j.aqrep.2023.101636.
-
Stoletov, K., Sanchez, S., Gorroño, I., Rabano, M., Vivanco, M. d. M., Kypta, R., and Lewis, J.D. (2023). Intravital imaging of Wnt/β-catenin and ATF2-dependent signalling pathways during tumour cell invasion and metastasis. Journal of Cell Science 136, jcs260285. 10.1242/jcs.260285.
-
Prosenz, K., Morice, S., Aguirre, J., Surdez, D., Votta-Velis, G., Borgeat, A., 2023. EP200 Impact of local anesthetics on bone sarcoma: an in vitro study, in: ePoster Session 6 – Station 4. Presented at the ESRA Abstracts, 40th Annual ESRA Congress, 6–9 September 2023, BMJ Publishing Group Ltd, pp. A149–A150. 10.1136/rapm-2023-ESRA.261
-
De Lima, J.M., Macedo, C.C.S., Barbosa, G.V., Castellano, L.R.C., Hier, M.P., Alaoui-Jamali, M.A., and Da Silva, S.D. (2023). E-liquid alters oral epithelial cell function to promote epithelial to mesenchymal transition and invasiveness in preclinical oral squamous cell carcinoma. Sci Rep 13, 3330. 10.1038/s41598-023-30016-0.
-
Skelin, J., Đukić, A., Filić, V., Hufbauer, M., Akgül, B., Thomas, M., Banks, L., and Tomaić, V. (2023). MAML1‐induced HPV E6 oncoprotein stability is required for cellular proliferation and migration of cervical tumor‐derived cells. Journal of Medical Virology 95, e28624. 10.1002/jmv.28624.
-
Kozhukharova, I., Minkevich, N., Alekseenko, L., Domnina, A. & Lyublinskaya, O. Paracrine and Autocrine Effects of VEGF Are Enhanced in Human eMSC Spheroids. IJMS 23, 14324 (2022).
-
Otręba, M., Stojko, J., Kabała‑Dzik, A., Rzepecka‑Stojko, A., 2022. Perphenazine and prochlorperazine decrease glioblastoma U‑87 MG cell migration and invasion: Analysis of the ABCB1 and ABCG2 transporters, E‑cadherin, α‑tubulin and integrins (α3, α5, and β1) levels. Oncol Lett 23, 182.
-
Greene, C.J., Anderson, S., Barthels, D., Howlader, M.S.I., Kanji, S., Sarkar, J., Das, H., 2022. DPSC Products Accelerate Wound Healing in Diabetic Mice through Induction of SMAD Molecules. Cells 11, 2409. https://doi.org/10.3390/cells11152409
-
Schnoell, J., Stanisz, I., Jank, B.J., Stanek, V., Schmid, R., Brunner, M., Heiduschka, G., Kotowski, U., 2022. Zerumbone acts as a radiosensitizer in head and neck squamous cell carcinoma. Invest New Drugs 40, 224–231. 10.1007/s10637-021-01190-7
-
Louro, A.F., Paiva, M.A., Oliveira, M.R., Kasper, K.A., Alves, P.M., Gomes‐Alves, P., and Serra, M. (2022). Bioactivity and miRNome Profiling of Native Extracellular Vesicles in Human Induced Pluripotent Stem Cell‐Cardiomyocyte Differentiation. Advanced Science 9, 2104296. https://doi.org/10.1002/advs.202104296.
-
Laubach, K.N., Yan, W., Kong, X., Sun, W., Chen, M., Zhang, J., and Chen, X. (2022). p73α1, a p73 C-terminal isoform, regulates tumor suppression and the inflammatory response via Notch1. Proc. Natl. Acad. Sci. U.S.A. 119, e2123202119. https://doi.org/10.1073/pnas.2123202119.
-
Solís-Fernández, G., Montero-Calle, A., Sánchez-Martínez, M., Peláez-García, A., Fernández-Aceñero, M.J., Pallarés, P., Alonso-Navarro, M., Mendiola, M., Hendrix, J., Hardisson, D., et al. (2022). Aryl-hydrocarbon receptor-interacting protein regulates tumorigenic and metastatic properties of colorectal cancer cells driving liver metastasis. Br J Cancer 126, 1604–1615. 10.1038/s41416-022-01762-1.
-
Gaertner, K., Tapanainen, R., Saari, S., Fekete, Z., Goffart, S., Pohjoismäki, J.L.O., Dufour, E., 2024. Exploring mitonuclear interactions in the regulation of cell physiology: insights from interspecies cybrids.
-
Miriam González González (2022). Host cell response to polymicrobial biofilms: implications in aspiration pneumonia., Jagiellonian University Krakow, Krakow
-
Anderson, S., Prateeksha, P., and Das, H. (2022). Dental Pulp-Derived Stem Cells Reduce Inflammation, Accelerate Wound Healing and Mediate M2 Polarization of Myeloid Cells. Biomedicines 10, 1999. 10.3390/biomedicines10081999.
-
Drljača, J., Popović, A., Bulajić, D., Stilinović, N., Vidičević Novaković, S., Sekulić, S., Milenković, I., Ninković, S., Ljubković, M., and Čapo, I. (2022). Diazepam diminishes temozolomide efficacy in the treatment of U87 glioblastoma cell line. CNS Neurosci Ther 28, 1447–1457. https://doi.org/10.1111/cns.13889.
-
Korona, B., Korona, D., Zhao, W., Wotherspoon, A.C., and Du, M.-Q. (2022). CCR6 activation links innate immune responses to MALT lymphoma development. Haematol.
-
Kirstein, A. (2022). Characterization of the radiation response of Glioblastoma cells with different radiosensitivities. Dissertation. Technische Universität München.
-
Haas, M., Lenz, T., Kadletz-Wanke, L., Heiduschka, G., Jank, B.J., 2022. The radiosensitizing effect of β-Thujaplicin, a tropolone derivative inducing S-phase cell cycle arrest, in head and neck squamous cell carcinoma-derived cell lines. Invest New Drugs. https://doi.org/10.1007/s10637-022-01229-3
-
Segelle, A., Núñez-Álvarez, Y., Oldfield, A.J., Webb, K.M., Voigt, P., Luco, R.F., 2022. Histone marks regulate the epithelial-to-mesenchymal transition via alternative splicing. Cell Reports 38, 110357. https://doi.org/10.1016/j.celrep.2022.110357
-
García-Jiménez, I., Cervantes-Villagrana, R.D., del-Río-Robles, J.E., Castillo-Kauil, A., Beltrán-Navarro, Y.M., García-Román, J., Reyes-Cruz, G., and Vázquez-Prado, J. (2022). Gβγ mediates activation of Rho guanine nucleotide exchange factor ARHGEF17 that promotes metastatic lung cancer progression. Journal of Biological Chemistry 298, 101440. 10.1016/j.jbc.2021.101440.
-
Arencibia, Alberto, Fernando Lanas, and Luis A. Salazar. 2022. Long Non-Coding RNAs Might Regulate Phenotypic Switch of Vascular Smooth Muscle Cells Acting as ceRNA: Implications for In-Stent Restenosis, International Journal of Molecular Sciences 23, no. 6: 3074. https://doi.org/10.3390/ijms23063074
-
Marzo, T. et al. (2022) Oxaliplatin inhibits angiogenin proliferative and cell migration effects in prostate cancer cells, Journal of Inorganic Biochemistry, 226, p. 111657. doi:10.1016/j.jinorgbio.2021.111657.
-
Anna Fliedner, 2021. Roles of transcriptional regulator PHF6 and transcriptional terminator SCAF4 in neurodevelopmental disorders. Friedrich-Alexander-Universität Erlangen-Nürnberg.
-
Koczurkiewicz-Adamczyk, P., Klaś, K., Gunia-Krzyżak, A., Piska, K., Andrysiak, K., Stępniewski, J., Lasota, S., Wójcik-Pszczoła, K., Dulak, J., Madeja, Z., Pękala, E., 2021. Cinnamic Acid Derivatives as Cardioprotective Agents against Oxidative and Structural Damage Induced by Doxorubicin. IJMS 22, 6217. 10.3390/ijms22126217
-
Reyner, C.L., Winter, R.L., Maneval, K.L., Boone, L.H., Wooldridge, A.A., 2021. Effect of recombinant equine interleukin-1β on function of equine endothelial colony-forming cells in vitro. ajvr 82, 318–325. https://doi.org/10.2460/ajvr.82.4.318
-
Karabicici, M., Azbazdar, Y., Ozhan, G., Senturk, S., Firtina Karagonlar, Z., Erdal, E., 2021. Changes in Wnt and TGF-β Signaling Mediate the Development of Regorafenib Resistance in Hepatocellular Carcinoma Cell Line HuH7. Front. Cell Dev. Biol. 9, 639779. 10.3389/fcell.2021.639779
-
Cheng, H.P., Huang, C.-J., Tsai, M.-L., Ong, H.T., Cheong, S.K., Choo, K.B., Chiou, S.-H., 2021. MicroRNA-362 negatively and positively regulates SMAD4 expression in TGF-β/SMAD signaling to suppress cell migration and invasion. Int. J. Med. Sci. 18, 1798–1809. 10.7150/ijms.50871
-
Rabbani, A., Haghniaz, R., Khan, T., Khan, R., Khalid, A., Naz, S.S., Ul-Islam, M., Vajhadin, F., Wahid, F., 2021. Development of bactericidal spinel ferrite nanoparticles with effective biocompatibility for potential wound healing applications. RSC Adv. 11, 1773–1782. 10.1039/D0RA08417D
-
Abuwarwar, M.H., Baker, A.T., Harding, J., Payne, N.L., Nagy, A., Knoblich, K., Fletcher, A.L., 2021. In Vitro Suppression of T Cell Proliferation Is a Conserved Function of Primary and Immortalized Human Cancer-Associated Fibroblasts. IJMS 22, 1827. https://doi.org/10.3390/ijms22041827
-
Biyik-Sit, R., Kruer, T., Dougherty, S., Bradley, J.A., Wilkey, D.W., Merchant, M.L., Trent, J.O., Clem, B.F., 2021. Nuclear Pyruvate Kinase M2 (PKM2) Contributes to Phosphoserine Aminotransferase 1 (PSAT1)-Mediated Cell Migration in EGFR-Activated Lung Cancer Cells. Cancers 13, 3938. https://doi.org/10.3390/cancers13163938
-
Kyriakopoulos, G., Katopodi, V., Skeparnias, I., Kaliatsi, E.G., Grafanaki, K., Stathopoulos, C., 2021. KRASG12C Can Either Promote or Impair Cap-Dependent Translation in Two Different Lung Adenocarcinoma Cell Lines. IJMS 22, 2222. https://doi.org/10.3390/ijms22042222
-
Elliott Grant Richards, 2021. [The role of decay accelerating factor in the pathogenesis of endometriosis (PhD thesis)](https://etd.ohiolink.edu/apexprod/rws_etd/send_file/send?accession=case1619689609524391. Li, Y., Shah, R.B., Sarti, S., Belcher, A.L., Lee, B.J., Gorbatenko, A., Nemati, F., Yu, H., Stanley, Z., Rahman, M., et al. (2023). A noncanonical IRAK4-IRAK1 pathway counters DNA damage–induced apoptosis independently of TLR/IL-1R signaling. Sci. Signal. 16, eadh3449. 10.1126/scisignal.adh3449.7&disposition=inline). Case Western Reserve University.
-
Bilotta, A.J. et al. (2021) Propionate Enhances Cell Speed and Persistence to Promote Intestinal Epithelial Turnover and Repair, Cellular and Molecular Gastroenterology and Hepatology, 11(4), pp. 1023–1044. doi:10.1016/j.jcmgh.2020.11.011.
-
Machado, S. et al. (2021) Toxicity in vitro and in Zebrafish Embryonic Development of Gold Nanoparticles Biosynthesized Using Cystoseira Macroalgae Extracts, International Journal of Nanomedicine, Volume 16, pp. 5017–5036. doi:10.2147/IJN.S300674.
-
Iwasaki, T., Onda, T., Honda, H., Hayashi, K., Shibahara, T., Nomura, T., and Takano, M. (2021). Over-expression of PDE5 in Oral Squamous Cell Carcinoma – Effect of Sildenafil Citrate. Anticancer Res 41, 2297–2306. 10.21873/anticanres.15005.
-
Paull, E.O. et al. (2021) A modular master regulator landscape controls cancer transcriptional identity, Cell, 184(2), pp. 334-351.e20. doi:10.1016/j.cell.2020.11.045.
-
Manuel Stefan Brugger. (2021). Sensorik, Aktorik und Stimulation vitaler Zellen unter Verwendung akustischer Oberflächenwellen. Dissertation, Universität Augsburg
-
Bownes, L.V. et al. (2021) EZH2 inhibition decreases neuroblastoma proliferation and in vivo tumor growth, PLOS ONE. Edited by J.W. Ramos, 16(3), p. e0246244. doi:10.1371/journal.pone.0246244.
-
Jain, M. et al. (2021) Integrin α9 regulates smooth muscle cell phenotype switching and vascular remodeling, JCI Insight, 6(10), p. e147134. doi:10.1172/jci.insight.147134.
-
Liedtke, V., Schröder, C., Roggenbuck, D., Weiss, R., Stohwasser, R., Schierack, P., Rödiger, S., and Schenk, L. (2021). LEDGF/p75 Is Required for an Efficient DNA Damage Response. IJMS 22, 5866. 10.3390/ijms22115866.
-
Wong, K.M., Song, J. and Wong, Y.H. (2021) CTCF and EGR1 suppress breast cancer cell migration through transcriptional control of Nm23-H1, Scientific Reports, 11(1), p. 491. doi:10.1038/s41598-020-79869-9.
-
Schnoell, J. et al. (2021) Zerumbone acts as a radiosensitizer in head and neck squamous cell carcinoma, Investigational New Drugs [Preprint]. doi:10.1007/s10637-021-01190-7.
-
Limaye, A.J., Bendzunas, G.N. and Kennedy, E.J. (2021) Targeted disruption of PKC from AKAP signaling complexes, RSC Chemical Biology, 2(4), pp. 1227–1231. doi:10.1039/D1CB00106J.
-
Erdem, Y.S. et al. (2021) An Image Segmentation Method for Wound Healing Assay Images, Natural and Applied Sciences Journal [Preprint]. doi:10.38061/idunas.853356.
-
Lee, J.H., Yoo, S.S., Hong, M.J., Choi, J.E., Kang, H., Do, S.K., Lee, W.K., Choi, S.H., Lee, Y.H., Seo, H., et al. (2021). Epigenetic readers and lung cancer: the rs2427964C>T variant of the bromodomain and extraterminal domain gene BRD3 is associated with poorer survival outcome in NSCLC. Mol Oncol 1878-0261.13109.
-
Rauschendorfer, T., Gurri, S., Heggli, I., Maddaluno, L., Meyer, M., Inglés-Prieto, Á., Janovjak, H., and Werner, S. (2021). Acute and chronic effects of a light-activated FGF receptor in keratinocytes in vitro and in mice. Life Sci. Alliance 4, e202101100.
-
Li, L., Mangali, S., Kour, N., Dasari, D., Ghatage, T., Sharma, V., Dhar, A., and Bhat, A. (2021). Syzygium cumini (jamun) fruit-extracted phytochemicals exert anti-proliferative effect on ovarian cancer cells. J Can Res Ther 0, 0.
-
Svanström, A., Rosendahl, J., Salerno, S., Leiva, M.C., Gregersson, P., Berglin, M., Bogestål, Y., Lausmaa, J., Oko, A., Chinga-Carrasco, G., et al. (2021). Optimized alginate-based 3D printed scaffolds as a model of patient derived breast cancer microenvironments in drug discovery. Biomed. Mater. 16, 045046.
-
Messner, C.J., Schmidt, S., Özkul, D., Gaiser, C., Terracciano, L., Krähenbühl, S., Suter-Dick, L., 2021. Identification of miR-199a-5p, miR-214-3p and miR-99b-5p as Fibrosis-Specific Extracellular Biomarkers and Promoters of HSC Activation. IJMS 22, 9799. https://doi.org/10.3390/ijms22189799
-
Dill, T.L., Carroll, A., Pinheiro, A., Gao, J., and Naya, F.J. (2021). The long noncoding RNA Meg3 regulates myoblast plasticity and muscle regeneration through epithelial-mesenchymal transition. Development 148, dev194027.
-
Wang, H., Li, B., Asha, K., Pangilinan, R.L., Thuraisamy, A., Chopra, H., Rokudai, S., Yu, Y., Prives, C.L., and Zhu, Y. (2021). The ion channel TRPM7 regulates zinc-depletion-induced MDMX degradation. Journal of Biological Chemistry 297, 101292. 10.1016/j.jbc.2021.101292.
-
Emma R Biglin (2021). DEVELOPMENT OF A BIO LOGICALLY- RELEVANT PRECLINICAL RADIOTHERAPY DOSIMETRY PHANTOM.
-
THERESA RAUSCHENDORFER (2021). MECHANISTIC ASSESSMENT OF FIBROBLAST GROWTH FACTOR RECEPTOR SIGNALING AND FUNCTION IN KERATINOCYTES.
-
Kauanova, S., Urazbayev, A., and Vorobjev, I. (2021). The Frequent Sampling of Wound Scratch Assay Reveals the “Opportunity” Window for Quantitative Evaluation of Cell Motility-Impeding Drugs. Front. Cell Dev. Biol. 9, 64097
-
Wezel, F., Lustig, J., Azoitei, A., Liu, J., Meessen, S., Najjar, G., Zehe, V., Faustmann, P., Zengerling, F., John, A., et al. (2021). Grainyhead-Like 3 Influences Migration and Invasion of Urothelial Carcinoma Cells. IJMS 22, 2959. 10.3390/ijms22062959.
-
Brugger, M.S., Schnitzler, L.G., Nieberle, T., Wixforth, A., and Westerhausen, C. (2021). Shear-horizontal surface acoustic wave sensor for non-invasive monitoring of dynamic cell spreading and attachment in wound healing assays. Biosensors and Bioelectronics 173, 112807.
-
Feiner, R. C. et al. EGFR-Binding Peptides: From Computational Design towards Tumor-Targeting of Adeno-Associated Virus Capsids. IJMS 21, 9535 (2020).
-
Gaba, R.C., Elkhadragy, L., Boas, F.E., Chaki, S., Chen, H.H., El-Kebir, M., Garcia, K.D., Giurini, E.F., Guzman, G., LoBianco, F.V., Neto, M.F., Newson, J.L., Qazi, A., Regan, M., Rund, L.A., Schwind, R.M., Stewart, M.C., Thomas, F.M., Whiteley, H.E., Wu, J., Schook, L.B., Schachtschneider, K.M., 2020. Development and comprehensive characterization of porcine hepatocellular carcinoma for translational liver cancer investigation. Oncotarget 11, 2686–2701. https://doi.org/10.18632/oncotarget.27647
-
Stope, M.B., Benouahi, R., Sander, C., Haralambiev, L., Nitsch, A., Egger, E., Mustea, A., 2020. Protherapeutic Effects and Inactivation of Mammary Carcinoma Cells by a Medical Argon Plasma Device. Anticancer Res 40, 6205–6212. 10.21873/anticanres.14640
-
Azar, W.J., Christie, E.L., Mitchell, C., Liu, D.S., Au-Yeung, G., Bowtell, D.D.L., 2020. Noncanonical IL6 Signaling-Mediated Activation of YAP Regulates Cell Migration and Invasion in Ovarian Clear Cell Cancer. Cancer Research 80, 4960–4971. https://doi.org/10.1158/0008-5472.CAN-19-3044
-
Pavlović, N., Calitz, C., Thanapirom, K., Mazza, G., Rombouts, K., Gerwins, P., and Heindryckx, F. (2020). Inhibiting IRE1α-endonuclease activity decreases tumor burden in a mouse model for hepatocellular carcinoma. ELife 9, e55865.
-
Bolf, E.L., Gillis, N.E., Davidson, C.D., Rodriguez, P.D., Cozzens, L., Tomczak, J.A., Frietze, S., and Carr, F.E. (2020). Thyroid Hormone Receptor Beta Induces a Tumor-Suppressive Program in Anaplastic Thyroid Cancer. Mol Cancer Res 18, 1443–1452.
-
Arildsen, N.S., and Hedenfalk, I. (2020). Simvastatin is a potential candidate drug in ovarian clear cell carcinomas. Oncotarget 11, 3660–3674.
-
Leclair, N.K., Brugiolo, M., Urbanski, L., Lawson, S.C., Thakar, K., Yurieva, M., George, J., Hinson, J.T., Cheng, A., Graveley, B.R., et al. (2020). Poison Exon Splicing Regulates a Coordinated Network of SR Protein Expression during Differentiation and Tumorigenesis. Molecular Cell 80, 648-665.e9.
-
Pucci, M., Gomes Ferreira, I., Malagolini, N., Ferracin, M., and Dall’Olio, F. (2020). The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition. IJMS 21, 6558.
-
Birladeanu, A., Rogalska, M., Potiri, M., Papadaki, V., Andreadou, M., Kontoyiannis, D., Erpapazoglou, Z., Lewis, J.D., and Kafasla, P. (2020). The IQGAP1-hnRNPM interaction links tumour-promoting alternative splicing to heat-induced signals (Molecular Biology).
-
Kodani, A., Kenny, C., Lai, A., Gonzalez, D.M., Stronge, E., Sejourne, G.M., Isacco, L., Partlow, J.N., O’Donnell, A., McWalter, K., et al. (2020). Posterior Neocortex-Specific Regulation of Neuronal Migration by CEP85L Identifies Maternal Centriole-Dependent Activation of CDK5. Neuron 106, 246-255.e6.
-
Issa, R., Thompson, K.L., and Price, B.L. (2022). Control of Staphylococcal-mediated endogenous protease activity alters wound closure time in a complex wound model. Journal of Dermatological Science 105, 105–112. 10.1016/j.jdermsci.2022.01.005.
-
Zegeye, M.M., Andersson, B., Sirsjö, A., and Ljungberg, L.U. (2020). IL-6 trans-Signaling Impairs Sprouting Angiogenesis by Inhibiting Migration, Proliferation and Tube Formation of Human Endothelial Cells. Cells 9, 1414.
-
Zubčić, V., Rinčić, N., Kurtović, M., Trnski, D., Musani, V., Ozretić, P., Levanat, S., Leović, D., and Sabol, M. (2020). GANT61 and Lithium Chloride Inhibit the Growth of Head and Neck Cancer Cell Lines Through the Regulation of GLI3 Processing by GSK3β. IJMS 21, 6410.
-
Zhou, Y., Chen, D., Xue, G., Yu, S., Yuan, C., Huang, M., Jiang, L., 2020. Improved therapeutic efficacy of quercetin-loaded polymeric nanoparticles on triple-negative breast cancer by inhibiting uPA. RSC Adv. 10, 34517–34526. 10.1039/D0RA04231E
-
Dashdulam, D., Kim, I.-D., Lee, H., Lee, H.-K., Kim, S.-W., and Lee, J.-K. (2020). Osteopontin heptamer peptide containing the RGD motif enhances the phagocytic function of microglia. Biochemical and Biophysical Research Communications 524, 371–377.
-
Wallace, R.G., Kenealy, M.-R., Brady, A.J., Twomey, L., Duffy, E., Degryse, B., Caballero-Lima, D., Moyna, N.M., Custaud, M.-A., Meade-Murphy, G., et al. (2020). Development of dynamic cell and organotypic skin models, for the investigation of a novel visco-elastic burns treatment using molecular and cellular approaches. Burns S0305417920303478.
-
Khong, Z.-J., Lai, S.-K., Koh, C.-G., Geifman-Shochat, S., and Li, H.-Y. (2020). A novel function of AAA-ATPase p97/VCP in the regulation of cell motility. Oncotarget 11, 74–85.
-
Azar, W.J., Christie, E.L., Mitchell, C., Liu, D.S., Au-Yeung, G., and Bowtell, D.D.L. (2020). Non-canonical IL-6 signaling-mediated activation of YAP regulates cell migration and invasion in ovarian clear cell cancer. Cancer Res canres.3044.2019.
-
Pucci, M., Gomes Ferreira, I., Orlandani, M., Malagolini, N., Ferracin, M., and Dall’Olio, F. (2020). High Expression of the Sda Synthase B4GALNT2 Associates with Good Prognosis and Attenuates Stemness in Colon Cancer. Cells 9, 948.
-
Lee, J., and Lee, W.R. (2021). [RNAi-based Gene Therapy Targeting ZGPAT Promotes EGF-dependent Wound Healing(https://emerginginvestigators.org/articles/21-095). J Emerg Invest. 10.59720/21-095.
-
Young, E.D., Manley, S.J., Beadnell, T.C., Shearin, A.E., Sasaki, K., Zimmerman, R., Kauffman, E., Vivian, C.J., Parasuram, A., Iwakuma, T., et al. (2021). Suppression of pancreatic cancer liver metastasis by secretion-deficient ITIH5. Br J Cancer 124, 166–175.
-
Badaoui, M., Zoso, A., Idris, T., Bacchetta, M., Simonin, J., Lemeille, S., Wehrle-Haller, B., and Chanson, M. (2020). Vav3 Mediates Pseudomonas aeruginosa Adhesion to the Cystic Fibrosis Airway Epithelium. Cell Reports 32, 107842.
-
Saenz de Viteri, M., Hernandez, M., Bilbao-Malavé, V., Fernandez-Robredo, P., González-Zamora, J., Garcia-Garcia, L., Ispizua, N., Recalde, S., and Garcia-Layana, A. (2020). A Higher Proportion of Eicosapentaenoic Acid (EPA) When Combined with Docosahexaenoic Acid (DHA) in Omega-3 Dietary Supplements Provides Higher Antioxidant Effects in Human Retinal Cells. Antioxidants 9, 828. 10.3390/antiox9090828.
-
Whittaker, T.E., Nagelkerke, A., Nele, V., Kauscher, U., and Stevens, M.M. (2020). Experimental artefacts can lead to misattribution of bioactivity from soluble mesenchymal stem cell paracrine factors to extracellular vesicles. Journal of Extracellular Vesicles 9, 1807674.
-
Sierra-Parraga, J.M., Merino, A., Eijken, M., Leuvenink, H., Ploeg, R., Møller, B.K., Jespersen, B., Baan, C.C., and Hoogduijn, M.J. (2020). Reparative effect of mesenchymal stromal cells on endothelial cells after hypoxic and inflammatory injury. Stem Cell Res Ther 11, 352.
-
Wierzbicki, M., Hotowy, A., Kutwin, M., Jaworski, S., Bałaban, J., Sosnowska, M., Wójcik, B., Wędzińska, A., Chwalibog, A., and Sawosz, E. (2020). Graphene Oxide Scaffold Stimulates Differentiation and Proangiogenic Activities of Myogenic Progenitor Cells. IJMS 21, 4173.
-
Pinnaratip, R. (2020). STUDY OF SILICA NANOPARTICLE COMPOSITE ON SILICA-HYDROGEN PEROXIDE COMPLEXATIONS AND THEIR EFFECTS IN CATECHOL BASED ADHESIVES. Doctor of Philosophy in Biomedical Engineering. Michigan Technological University.
-
Semina, E.V., Rubina, K.A., Shmakova, A.A., Rysenkova, K.D., Klimovich, P.S., Aleksanrushkina, N.A., Sysoeva, V.Y., Karagyaur, M.N., and Tkachuk, V.A. (2020). Downregulation of uPAR promotes urokinase translocation into the nucleus and epithelial to mesenchymal transition in neuroblastoma. J Cell Physiol 235, 6268–6286.
-
Suarez-Arnedo, A., Torres Figueroa, F., Clavijo, C., Arbeláez, P., Cruz, J.C., and Muñoz-Camargo, C. (2020). An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 15, e0232565.
-
Lorelai L. Castro, Chinah Noelle, D. Torres, and Romeric F. Pobre (2020). Effects of Schumann’s Resonant Frequencies on In-Vitro Normal Human Dermal Fibroblast (NHDF) Cells Using Pulsed Electromagnetic Field (PEMF). DLSU Research Congress 2020 Building Resilient, Innovative, and Sustainable Societies.
-
Gomez-Auli, A., Hillebrand, L.E., Christen, D., Günther, S.C., Biniossek, M.L., Peters, C., Schilling, O., and Reinheckel, T. (2020). The secreted inhibitor of invasive cell growth CREG1 is negatively regulated by cathepsin proteases. Cell. Mol. Life Sci.
-
Tam, S.Y., Wu, V.W.C., and Law, H.K.W. (2020). JNK Pathway Mediates Low Oxygen Level Induced Epithelial–Mesenchymal Transition and Stemness Maintenance in Colorectal Cancer Cells. Cancers 12, 224.
-
Carlsson, E., Supharattanasitthi, W., Jackson, M., and Paraoan, L. (2020). Increased Rate of Retinal Pigment Epithelial Cell Migration and Pro-Angiogenic Potential Ensuing From Reduced Cystatin C Expression. Invest. Ophthalmol. Vis. Sci. 61, 9.
-
Ambreen, Ghazala, et al. Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes. Cancers, vol. 12, no. 11, 2020
-
Fliedner, A., Gregor, A., Ferrazzi, F., Ekici, A.B., Sticht, H., Zweier, C., 2020. Loss of PHF6 leads to aberrant development of human neuron-like cells. Sci Rep 10, 19030. https://doi.org/10.1038/s41598-020-75999-2
-
Ng, A.H.M., Khoshakhlagh, P., Rojo Arias, J.E., Pasquini, G., Wang, K., Swiersy, A., Shipman, S.L., Appleton, E., Kiaee, K., Kohman, R.E., et al. (2021). A comprehensive library of human transcription factors for cell fate engineering. Nat Biotechnol 39, 510–519. 10.1038/s41587-020-0742-6.
-
Nguyen, H.M.-H., Torres, J.A., Agrawal, S., and Agrawal, A. (2020). Nicotine Impairs the Response of Lung Epithelial Cells to IL-22. Mediators of Inflammation, 2020, 1–9.
-
Li, P., Butcher, N.J., and Minchin, R.F. (2020). Effect arylamine N-acetyltransferase 1 on morphology, adhesion, migration, and invasion of MDA-MB-231 cells: role of matrix metalloproteinases and integrin αV. Cell Adhesion & Migration 14, 1–11.
-
Hámori, L., Kudlik, G., Szebényi, K., Kucsma, N., Szeder, B., Póti, Á., Uher, F., Várady, G., Szüts, D., Tóvári, J., et al. (2020). Establishment and Characterization of a Brca1−/−, p53−/− Mouse Mammary Tumor Cell Line. IJMS 21, 1185.
-
Jain, M., Dhanesha, N., Doddapattar, P., Chorawala, M.R., Nayak, M.K., Cornelissen, A., Guo, L., Finn, A.V., Lentz, S.R., and Chauhan, A.K. (2019). Smooth muscle cell–specific fibronectin-EDA mediates phenotypic switching and neointimal hyperplasia. Journal of Clinical Investigation 130, 295–314.
-
Fan, T. S. (2019). Herpesvirus-Encoded G Protein-Coupled Receptor Signaling and its role in the Modulation of Glioblastoma Multiforme. PhD-Thesis - Research and graduation internal, Vrije Universiteit Amsterdam
-
Barson, E., 2019. Differential expression of the tight junction proteins occludin, ZO1 and ZO2 with respects to the progression of liver diseases (phD). Coventry University, Coventry.
-
Li, L., Ma, L., Wang, D., Jia, H., Yu, M., Gu, Y., Shang, H., and Zou, Z. (2019). Design and Synthesis of Matrine Derivatives as Novel Anti-Pulmonary Fibrotic Agents via Repression of the TGFβ/Smad Pathway. Molecules 24, 1108. 10.3390/molecules24061108.
-
Saidu, N.E.B., Filić, V., Thomas, M., Sarabia-Vega, V., Đukić, A., Miljković, F., Banks, L., and Tomaić, V. (2019). PDZ Domain-Containing Protein NHERF-2 Is a Novel Target of Human Papillomavirus 16 (HPV-16) and HPV-18. J Virol 94, e00663-19, /jvi/94/1/JVI.00663-19.atom.
-
Caseiro, A.R., Santos Pedrosa, S., Ivanova, G., Vieira Branquinho, M., Almeida, A., Faria, F., Amorim, I., Pereira, T., and Maurício, A.C. (2019). Mesenchymal Stem/ Stromal Cells metabolomic and bioactive factors profiles: A comparative analysis on the umbilical cord and dental pulp derived Stem/ Stromal Cells secretome. PLoS ONE 14, e0221378.
-
Li, J., Cheng, D., Zhu, M., Yu, H., Pan, Z., Liu, L., Geng, Q., Pan, H., Yan, M., and Yao, M. (2019). OTUB2 stabilizes U2AF2 to promote the Warburg effect and tumorigenesis via the AKT/mTOR signaling pathway in non-small cell lung cancer. Theranostics 9, 179–195.
-
Bloch, E., Sikorski, E.L., Pontoriero, D., Day, E.K., Berger, B.W., Lazzara, M.J., and Thévenin, D. (2019). Disrupting the transmembrane domain–mediated oligomerization of protein tyrosine phosphatase receptor J inhibits EGFR-driven cancer cell phenotypes. J. Biol. Chem. 294, 18796–18806.
-
Johanna De Vestele (2019). MEASURING THE CELLULAR RESPONSE OF MEAT-RELATED COMPONENTS ON HCT-COLON CANCER CELLS USING IN VITRO ASSAYS. Universiteit Gent.
-
Samantha Hain (2019). [The Roles of CXCR4 and CXCR7 in Melanocyte and Melanoma Motility](http://scholarworks.csun.edu/handle/10211.3/213151. Master of Science in Biology). CALIFORNIA STATE UNIVERSITY.
-
Harmati, M., Gyukity-Sebestyen, E., Dobra, G., Janovak, L., Dekany, I., Saydam, O., Hunyadi-Gulyas, E., Nagy, I., Farkas, A., Pankotai, T., et al. (2019). Small extracellular vesicles convey the stress-induced adaptive responses of melanoma cells. Sci Rep 9, 15329.
-
Hashimoto, Y., Shiina, M., Dasgupta, P., Kulkarni, P., Kato, T., Wong, R.K., Tanaka, Y., Shahryari, V., Maekawa, S., Yamamura, S., et al. (2019). Upregulation of miR-130b Contributes to Risk of Poor Prognosis and Racial Disparity in African-American Prostate Cancer. Cancer Prev Res 12, 585–598.
-
Jain, M., Dhanesha, N., Doddapattar, P., Chorawala, M.R., Nayak, M.K., Cornelissen, A., Guo, L., Finn, A.V., Lentz, S.R., and Chauhan, A.K. (2019). Smooth muscle cell–specific fibronectin-EDA mediates phenotypic switching and neointimal hyperplasia. Journal of Clinical Investigation 130, 295–314.
-
Kim, K.Y., Yoon, M., Cho, Y., Lee, K.-H., Park, S., Lee, S., Choi, S.-Y., Lee, D., Yang, C., Cho, E.H., et al. (2019). Targeting metastatic breast cancer with peptide epitopes derived from autocatalytic loop of Prss14/ST14 membrane serine protease and with monoclonal antibodies. J Exp Clin Cancer Res 38, 363.
-
Pinto, R.V., Fernandes, A.C., Antunes, F., Lin, Z., Rocha, J., Pires, J., and Pinto, M.L. (2019). "New generation of nitric oxide-releasing porous materials: Assessment of their potential to regulate biological functions":https://www.sciencedirect.com/science/article/abs/pii/S1089860319300436. Nitric Oxide 90, 29–36.
-
Torrens-Mas, M., Hernández-López, R., Pons, D.-G., Roca, P., Oliver, J., and Sastre-Serra, J. (2019). Sirtuin 3 silencing impairs mitochondrial biogenesis and metabolism in colon cancer cells. American Journal of Physiology-Cell Physiology 317, C398–C404.
-
Fink, A.F., Ciliberti, G., Popp, R., Sirait-Fischer, E., Frank, A.-C., Fleming, I., Sekar, D., Weigert, A., and Brüne, B. (2019). IL27Rα Deficiency Alters Endothelial Cell Function and Subverts Tumor Angiogenesis in Mammary Carcinoma. Front. Oncol. 9, 1022.
-
Juárez-Cruz, J.C., Zuñiga-Eulogio, M.D., Olea-Flores, M., Castañeda-Saucedo, E., Mendoza-Catalán, M.Á., Ortuño-Pineda, C., Moreno-Godínez, M.E., Villegas-Comonfort, S., Padilla-Benavides, T., and Navarro-Tito, N. (2019). Leptin induces cell migration and invasion in a FAK-Src- dependent manner in breast cancer cells, Cell Biology.
-
Persson, S.T., Ekström, S., Papareddy, P., and Herwald, H. (2019). Cold Atmospheric Plasma Disarms M1 Protein, an Important Streptococcal Virulence Factor. J Innate Immun 1–14.
-
Gong, S., Qu, X., Yang, S., Zhou, S., Li, P., and Zhang, Q. (2019). RFC3 induces epithelial‑mesenchymal transition in lung adenocarcinoma cells through the Wnt/β‑catenin pathway and possesses prognostic value in lung adenocarcinoma. Int J Mol Med.
-
Roca-Lema, D., Martinez-Iglesias, O., Portela, C.F. de A., Rodríguez-Blanco, A., Valladares-Ayerbes, M., Díaz-Díaz, A., Casas-Pais, A., Prego, C., and Figueroa, A. (2019). In Vitro Anti-proliferative and Anti-invasive Effect of Polysaccharide-rich Extracts from Trametes Versicolor and Grifola Frondosa in Colon Cancer Cells. Int. J. Med. Sci. 16, 231–240.
-
Altemus, M.A., Goo, L.E., Little, A.C., Yates, J.A., Cheriyan, H.G., Wu, Z.F., and Merajver, S.D. (2019). Breast cancers utilize hypoxic glycogen stores via PYGB, the brain isoform of glycogen phosphorylase, to promote metastatic phenotypes. PLoS ONE 14, e0220973.
-
Aijaz, A., Teryek, M., Goedken, M., Polunas, M., and Olabisi, R.M. (2019). Coencapsulation of ISCs and MSCs Enhances Viability and Function of both Cell Types for Improved Wound Healing. Cel. Mol. Bioeng. 12, 481–493.
-
Garivet, G., Hofer, W., Konitsiotis, A., Klein, C., Kaiser, N., Mejuch, T., Fansa, E., Alsaabi, R., Wittinghofer, A., Bastiaens, P.I.H., et al. (2019). Small-Molecule Inhibition of the UNC-Src Interaction Impairs Dynamic Src Localization in Cells. Cell Chemical Biology 26, 842-851.e7.
-
Marques, M.C., Albuquerque, I.S., Vaz, S.H., and Bernardes, G.J.L. (2019). Overexpression of osmosensitive Ca 2+-activated channel TMEM63B promotes migration in HEK293T cells, Biochemistry.
-
Alexandre Segelle. The role of histone modifications in the regulation of alternative splicing during epithelial-to-mesenchymal transition. Agricultural sciences. Université Montpellier, 2020. English. ⟨NNT : 2020MONTT017⟩. ⟨tel-03137009⟩
-
Goels, T., Eichenauer, E., Langeder, J., Hoeller, F., Sykora, C., Tahir, A., Urban, E., Heiss, E.H., Saukel, J., and Glasl, S. (2020). Norway Spruce Balm: Phytochemical Composition and Ability to Enhance Re-epithelialization In Vitro. Planta Med 86, 1080–1088. 10.1055/a-1141-0921.
-
Villatoro, A.J., Alcoholado, C., Martín-Astorga, M. del C., Rico, G., Fernández, V., and Becerra, J. (2020). Characterization of the secretory profile and exosomes of limbal stem cells in the canine species. PLoS ONE 15, e0244327.
-
Carvajal, F., Duran, C., and Aquea, F. (2020). Effect of alerce ( Fitzroya cupressoides) cell culture extract on wound healing repair in a human keratinocyte cell line. J Cosmet Dermatol 19, 1254–1259. 10.1111/jocd.13137.
-
Rocha, M.I., Dias, F., Resende, M., Sousa, M., Duarte, M., Tomás, A.M., Castro, H., 2020. Leishmania infantum Enhances Migration of Macrophages via a Phosphoinositide 3-Kinase γ-Dependent Pathway. ACS Infect. Dis. 6, 1643–1649. https://doi.org/10.1021/acsinfecdis.0c00080
-
Kodani, A., Kenny, C., Lai, A., Gonzalez, D.M., Stronge, E., Sejourne, G.M., Isacco, L., Partlow, J.N., O’Donnell, A., McWalter, K., et al. (2020). Posterior Neocortex-Specific Regulation of Neuronal Migration by CEP85L Identifies Maternal Centriole-Dependent Activation of CDK5. Neuron 106, 246-255.e6.
-
Capdevielle, C., Desplat, A., Charpentier, J., Saggliocco, F., Thiebaud, P., Thézé, N., Fédou, S., Hooks, K.B., Silvestri, R., Guyonnet-Duperat, V., et al. (2019). HDAC inhibition induces expression of scaffolding proteins critical for tumor progression in pediatric glioma: focus on EBP50 and IRSp53. Neuro-Oncology noz215.
-
Íris Cláudia Felisberto Guerreiro, 2019. Exploring new therapeutic targets and novel therapies for resistant colorectal cancer subtypes. Universidade Nova de Lisboa.
-
Lundby, A., Franciosa, G., Emdal, K.B., Refsgaard, J.C., Gnosa, S.P., Bekker-Jensen, D.B., Secher, A., Maurya, S.R., Paul, I., Mendez, B.L., et al. (2019). Oncogenic Mutations Rewire Signaling Pathways by Switching Protein Recruitment to Phosphotyrosine Sites. Cell 179, 543-560.e26.
-
Davis, J.B., Krishna, S.S., Abi Jomaa, R., Duong, C.T., Espina, V., Liotta, L.A., and Mueller, C. (2019). A new model isolates glioblastoma clonal interactions and reveals unexpected modes for regulating motility, proliferation, and drug resistance. Sci Rep 9, 17380.
-
Pavlovic, N., Thanapirom, K., Mazza, G., Rombouts, K., Gerwins, P., and Heindryckx, F. (2019). Blocking IRE1a;-endoribonuclease activity in hepatic stellate cells decreases tumor cell proliferation and metastasis in hepatocellular carcinoma, Cancer Biology.
-
Michel, M., Hollenbach, M., Pohl, S., Ripoll, C., and Zipprich, A. (2019). Inhibition of Glyoxalase-I Leads to Reduced Proliferation, Migration and Colony Formation, and Enhanced Susceptibility to Sorafenib in Hepatocellular Carcinoma. Front. Oncol. 9, 785.
-
Yasi, E.A., Allen, A.A., Sugianto, W., and Peralta-Yahya, P. (2019). Identification of Three Antimicrobials Activating Serotonin Receptor 4 in Colon Cells. ACS Synth. Biol. acssynbio.9b00310.
-
Marzoq, A.J., Mustafa, S.A., Heidrich, L., Hoheisel, J.D., and Alhamdani, M.S.S. (2019). Impact of the secretome of activated pancreatic stellate cells on growth and differentiation of pancreatic tumour cells. Sci Rep 9, 5303.
-
Schäfer, A., Gjerga, E., Welford, R.W., Renz, I., Lehembre, F., Groenen, P.M., Saez‐Rodriguez, J., Aebersold, R., and Gstaiger, M. (2019). Elucidating essential kinases of endothelin signalling by logic modelling of phosphoproteomics data. Mol Syst Biol 15.
-
Venturi, G., Gomes Ferreira, I., Pucci, M., Ferracin, M., Malagolini, N., Chiricolo, M., and Dall’Olio, F. (2019). Impact of sialyltransferase ST6GAL1 overexpression on different colon cancer cell types. Glycobiology 29, 684–695.
-
Fang, L.-W., Kao, Y.-H., Chuang, Y.-T., Huang, H.-L., and Tai, T.-S. (2019). Ets-1 enhances tumor migration through regulation of CCR7 expression. BMB Rep. 52, 548–553.
-
Fenu, M., Bettermann, T., Vogl, C., Darwish-Miranda, N., Schramel, J., Jenner, F., and Ribitsch, I. (2019). A novel magnet-based scratch method for standardisation of wound-healing assays. Sci Rep 9, 12625.
-
Abdallah, S., Abu-Reidah, I., Mousa, A., and Abdel-Latif, T. (2019). Rhus coriaria (sumac) extract reduces migration capacity of uterus cervix cancer cells., Revista Brasileira de Farmacognosia S0102695X19300547.
-
Yagnik, G., Rutowski, M.J., Shah, S.S., and Aghi, M.K. (2019). Stratifying nonfunctional pituitary adenomas into two groups distinguished by macrophage subtypes. Oncotarget 10.
-
Bashari, M.H., Huda, F., Tartila, T.S., Shabrina, S., Putri, T., Qomarilla, N., Atmaja, H., Subhan, B., Sudji, I.R., and Meiyanto, E. (2019). Bioactive Compounds in the Ethanol Extract of Marine Sponge Stylissa carteri Demonstrates Potential Anti-Cancer Activity in Breast Cancer Cells. Asian Pac J Cancer Prev 20, 1199–1206.
-
Stafman, L.L., Waldrop, M.G., Williams, A.P., Aye, J.M., Stewart, J.E., Mroczek-Musulman, E., Yoon, K.J., Whelan, K., and Beierle, E.A. (2019). The Presence of PIM3 Increases Hepatoblastoma Tumorigenesis and Tumor Initiating Cell Phenotype and is Associated with Decreased Patient Survival. Journal of Pediatric Surgery.
-
Schacke, M., Kumar, J., Colwell, N., Hermanson, K., Folle, G., Nechaev, S., Dhasarathy, A., and Lafon-Hughes, L. (2019). PARP-1/2 Inhibitor Olaparib Prevents or Partially Reverts EMT Induced by TGF-β in NMuMG Cells. International Journal of Molecular Sciences 20, 518.
-
Botelho, C.M., Gonçalves, O., Marques, R., Thiagarajan, V., Vorum, H., Gomes, A.C., and Neves-Petersen, M.T. (2018). Photonic modulation of epidermal growth factor receptor halts receptor activation and cancer cell migration. J. Biophotonics 11, e201700323.
-
Chai, C., Wu, H., Wang, B., Eisenstat, D.D., Leng, R.P., 2018. MicroRNA-498 promotes proliferation and migration by targeting the tumor suppressor PTEN in breast cancer cells. Carcinogenesis 39, 1185–1196. 10.1093/carcin/bgy092
-
Islam, M.R. (2018). Is it all just an Akt - you’d be SMAD to believe it! Role of TGFβ1 in oral cancer metastasis. Science Repository Oü.
-
Erica Hawkins, 2018. Exploring Plant Stilbenes For Healthy Skin. University of East Anglia.
-
Bhattacharya A., Kumar J., Hermanson K., Sun Y., Qureshi H., Perley D., Scheidegger A., Singh B. B., Dhasarathy A. The calcium channel proteins ORAI3 and STIM1 mediate TGF-β induced Snai1 expression. Oncotarget. 2018; 9: 29468-29483.
-
Brissos, R.F., Clavero, P., Gallen, A., Grabulosa, A., Barrios, L.A., Caballero, A.B., Korrodi-Gregório, L., Pérez-Tomás, R., Muller, G., Soto-Cerrato, V., et al. (2018). Highly Cytotoxic Ruthenium(II)-Arene Complexes from Bulky 1-Pyrenylphosphane Ligands. Inorg. Chem. 57, 14786–14797.
-
Lasiste, J.M., Zoroquiain, P., Miyamoto, D., and Burnier, M. (2018). Metformin activity in an in vitro model of posterior capsule opacification. Vision Pan-America, The Pan-American Journal of Ophthalmology 17, 105–112.
-
Jessica Warrington (2018). The Role of Receptor Activity Modifying Protein 1 in Prostate Cancer. PhD thesis. University of Sheffield.
-
Asiri, A., Toss, M.S., Raposo, T.P., Akhlaq, M., Thorpe, H., Alfahed, A., Asiri, A., and Ilyas, M. (2018). The Cten signalling pathway stabilises Src protein to promote Epithelial-Mesenchymal Transition (EMT) in colorectal cancer. BioRxiv.
-
Paul Grevitt, 2018. Identification of novel regulators of HIFs for use in anti-cancer target development. Queen Mary University of London.
-
Rogers, S., McCloy, R.A., Parker, B.L., Gallego-Ortega, D., Law, A.M.K., Chin, V.T., Conway, J.R.W., Fey, D., Millar, E.K.A., O’Toole, S., et al. (2018). MASTL overexpression promotes chromosome instability and metastasis in breast cancer. Oncogene 37, 4518–4533.
-
Anne Lewis Carlton (2018). The role of CBFβ in ovarian cancer. University of Virginia.
-
Alan Lovelace, 2018. The Cellular Growth Analyzer: A Simpler and More Comprehensive Scratch Assay Analyzing Program (Master Thesis). Texas Tech University.
-
Walerych, D., Pruszko, M., Zyla, L., Wezyk, M., Gaweda-Walerych, K., and Zylicz, A. (2018). Wild-type p53 oligomerizes more efficiently than p53 hot-spot mutants and overcomes mutant p53 gain-of-function via a "dominant-positive" mechanism. Oncotarget 9.
-
Accornero, P., Martignani, E., Miretti, S., and Baratta, M. (2018). Murine and Human Mammary Cancer Cell Lines: Functional Tests. In Epithelial Cell Culture, M. Baratta, ed. (New York, NY: Springer New York), pp. 169–183.
-
Daniel, B., Nagy, G., Czimmerer, Z., Horvath, A., Hammers, D.W., Cuaranta-Monroy, I., Poliska, S., Tzerpos, P., Kolostyak, Z., Hays, T.T., et al. (2018). The Nuclear Receptor PPARγ Controls Progressive Macrophage Polarization as a Ligand-Insensitive Epigenomic Ratchet of Transcriptional Memory. Immunity 49, 615-626.e6.
-
Carlton, A.L., Illendula, A., Gao, Y., Llaneza, D.C., Boulton, A., Shah, A., Rajewski, R.A., Landen, C.N., Wotton, D., and Bushweller, J.H. (2018). Small molecule inhibition of the CBFβ/RUNX interaction decreases ovarian cancer growth and migration through alterations in genes related to epithelial-to-mesenchymal transition. Gynecologic Oncology 149, 350–360.
-
Stamm, A., Strauß, S., Vogt, P., Scheper, T., and Pepelanova, I. (2018). Positive in vitro wound healing effects of functional inclusion bodies of a lipoxygenase from the Mexican axolotl. Microbial Cell Factories 17.
-
Whittaker, T.E., 2018. Investigating exosomes for regenerative medicine: development of purification techniques and novel analytical strategies for assessment of potency. https://doi.org/10.25560/89473
-
Berning, P., Schaefer, C., Clemens, D., Korsching, E., Dirksen, U., and Potratz, J. (2018). The CXCR4 antagonist plerixafor (AMD3100) promotes proliferation of Ewing sarcoma cell lines in vitro and activates receptor tyrosine kinase signaling. Cell Communication and Signaling 16.
-
Prestigiacomo, V., and Suter-Dick, L. (2018). Nrf2 protects stellate cells from Smad-dependent cell activation. PLOS ONE 13, e0201044.
-
Leifheit-Nestler, M., Kirchhoff, F., Nespor, J., Richter, B., Soetje, B., Klintschar, M., Heineke, J., and Haffner, D. (2018). Fibroblast growth factor 23 is induced by an activated renin–angiotensin–aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrology Dialysis Transplantation, 33, 1722–1734.
-
Hopkins, B.L., Nadler, M., Skoko, J.J., Bertomeu, T., Pelosi, A., Shafaei, P.M., Levine, K., Schempf, A., Pennarun, B., Yang, B., et al. (2018). A Peroxidase Peroxiredoxin 1-Specific Redox Regulation of the Novel FOXO3 microRNA Target let-7. Antioxidants & Redox Signaling 28, 62–77.
-
Saini, M., Verma, A., and Mathew, S.J. (2018). SPRY2 is a novel MET interactor that regulates metastatic potential and differentiation in rhabdomyosarcoma. Cell Death & Disease 9.
-
Persson, S.T., Hauri, S., Malmström, J., and Herwald, H. (2018). Leucocyte recruitment and molecular fortification of keratinocytes triggered by streptococcal M1 protein. Cellular Microbiology 20, e12792.
-
Fung, E., Richter, C., Yang, H., Schäffer, I., Fischer, R., Kessler, B.M., Bassermann, F., and D’Angiolella, V. (2018). FBXL13 directs the proteolysis of CEP192 to regulate centrosome homeostasis and cell migration. EMBO Reports e44799.
-
Rianna, C., and Radmacher, M. (2017). Influence of microenvironment topography and stiffness on the mechanics and motility of normal and cancer renal cells. Nanoscale 9, 11222–11230.
-
Fernandez-Gutierrez, M.M., Roosjen, P.P.J., Ultee, E., Agelink, M., Vervoort, J.J.M., Keijser, B., Wells, J.M., Kleerebezem, M., 2017. Streptococcus salivarius MS-oral-D6 promotes gingival re-epithelialization in vitro through a secreted serine protease. Sci Rep 7, 11100. 10.1038/s41598-017-11446-z
-
Barhanpurkar-Naik, A., Mhaske, S.T., Pote, S.T., Singh, K., Wani, M.R., 2017. Interleukin-3 enhances the migration of human mesenchymal stem cells by regulating expression of CXCR4. Stem Cell Res Ther 8, 168. 10.1186/s13287-017-0618-y
-
Hauck, P.M., Wolf, E.R., Olivos, D.J., Batuello, C.N., McElyea, K.C., McAtarsney, C.P., Cournoyer, R.M., Sandusky, G.E., and Mayo, L.D. (2017). Early-Stage Metastasis Requires Mdm2 and Not p53 Gain of Function. Molecular Cancer Research 15, 1598–1607.
-
Sun, X., Wang, S.C., Wei, Y., Luo, X., Jia, Y., Li, L., Gopal, P., Zhu, M., Nassour, I., Chuang, J.-C., et al. (2017). Arid1a Has Context-Dependent Oncogenic and Tumor Suppressor Functions in Liver Cancer. Cancer Cell 32, 574–589.e6.
-
Castañón, E., Soltermann, A., López, I., Román, M., Ecay, M., Collantes, M., Redrado, M., Baraibar, I., López-Picazo, J.M., Rolfo, C., et al. (2017). The inhibitor of differentiation-1 ( Id1 ) enables lung cancer liver colonization through activation of an EMT program in tumor cells and establishment of the pre-metastatic niche. Cancer Letters 402, 43–51.
-
Jade Marie Edenvirg Fontanilla Lasiste (2017). METFORMIN INHIBITS EPITHELIAL-TO-MESENCHYMAL TRANSITION IN LENS EPITHELIAL CELLS. Master of Science (MSc) in Pathology. McGill University.
-
Hauck, P.M., Wolf, E.R., Olivos, D.J., Batuello, C.N., McElyea, K.C., McAtarsney, C.P., Cournoyer, R.M., Sandusky, G.E., and Mayo, L.D. (2017). Early-Stage Metastasis Requires Mdm2 and Not p53 Gain of Function. Molecular Cancer Research 15, 1598–1607.
-
Calvo, N., Carriere, P., Martin, M.J., and Gentili, C. (2017). RSK activation via ERK modulates human colon cancer cells response to PTHrP. Journal of Molecular Endocrinology 59, 13–27.
-
Duanduan Cong (2017). Identification of functional single nucleotide polymorphisms (SNPs) in High Risk-Human Papillomavirus (HR-HPV) related diseases. The University of Edinburgh.
-
Auer, S., Rinnerthaler, M., Bischof, J., Streubel, M.K., Breitenbach-Koller, H., Geisberger, R., Aigner, E., Cadamuro, J., Richter, K., Sopjani, M., et al. (2017). The Human NADPH Oxidase, Nox4, Regulates Cytoskeletal Organization in Two Cancer Cell Lines, HepG2 and SH-SY5Y. Frontiers in Oncology 7.
-
Kabała-Dzik, A., Rzepecka-Stojko, A., Kubina, R., Jastrzębska-Stojko, Ż., Stojko, R., Wojtyczka, R., and Stojko, J. (2017). Migration Rate Inhibition of Breast Cancer Cells Treated by Caffeic Acid and Caffeic Acid Phenethyl Ester: An In Vitro Comparison Study. Nutrients 9, 1144.
-
Juneja, M., Kobelt, D., Walther, W., Voss, C., Smith, J., Specker, E., Neuenschwander, M., Gohlke, B.-O., Dahlmann, M., Radetzki, S., et al. (2017). Statin and rottlerin small-molecule inhibitors restrict colon cancer progression and metastasis via MACC1. PLOS Biology 15, e2000784.
-
Hoyle, N.P., Seinkmane, E., Putker, M., Feeney, K.A., Krogager, T.P., Chesham, J.E., Bray, L.K., Thomas, J.M., Dunn, K., Blaikley, J., et al. (2017). Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci. Transl. Med. 9, eaal2774.
-
Morry, J., Ngamcherdtrakul, W., Gu, S., Reda, M., Castro, D.J., Sangvanich, T., Gray, J.W., and Yantasee, W. (2017). Targeted Treatment of Metastatic Breast Cancer by PLK1 siRNA Delivered by an Antioxidant Nanoparticle Platform. Molecular Cancer Therapeutics 16, 763–772.
-
Qiao, Y., Chen, J., Lim, Y.B., Finch-Edmondson, M.L., Seshachalam, V.P., Qin, L., Jiang, T., Low, B.C., Singh, H., Lim, C.T., et al. (2017). YAP Regulates Actin Dynamics through ARHGAP29 and Promotes Metastasis. Cell Reports 19, 1495–1502.
-
Mahendra, A., Yang, X., Abnouf, S., Park, D., Soomro, S., Adolacion, J.R.T., Roszik, J., Coarfa, C., Romain, G., Wanzeck, K., et al. (2017). Beyond Autoantibodies: Biological Roles Of Human Autoreactive B Cells In Rheumatoid Arthritis Revealed By Whole Transcriptome Profiling. preprint
-
Ottosson, M., Jakobsson, A., and Johansson, F. (2017). Accelerated Wound Closure - Differently Organized Nanofibers Affect Cell Migration and Hence the Closure of Artificial Wounds in a Cell Based In Vitro Model.. PLOS ONE 12, e0169419.
-
Buesch, S., Schaepermeier, S., D’Souza, T., Ortmann, B., Schwartz, C., and Schroeder, J. (2017). Abstract 822: Simple and easy monitoring of tube formation and migration assays with the CytoSMART TM Live Cell Imaging System.. Cancer Research 77, 822–822. See also this poster
-
Walter, Thomas M. & Merish, S. (2016). In-Vitro wound healing activity of Herbal topical formulation on H9C2 Heart cells. Siddha Papers 0974-2522. 2016(1). 1-11.
-
Saenz-de-Viteri, M., Fernández-Robredo, P., Hernández, M., Bezunartea, J., Reiter, N., Recalde, S., and García-Layana, A. (2016). Single- and repeated-dose toxicity study of bevacizumab, ranibizumab, and aflibercept in ARPE-19 cells under normal and oxidative stress conditions. Biochemical Pharmacology 103, 129–139.
-
Yong, L.-K., Lai, S., Liang, Z., Poteet, E., Chen, F., van Buren, G., Fisher, W., Mo, Q., Chen, C., and Yao, Q. (2016). Overexpression of Semaphorin-3E enhances pancreatic cancer cell growth and associates with poor patient survival. Oncotarget 7.
-
Urello, M.A., Kiick, K.L., and Sullivan, M.O. (2016). Integration of growth factor gene delivery with collagen-triggered wound repair cascades using collagen-mimetic peptides. Urello et al. Bioengineering & Translational Medicine 1, 207–219.
-
Touat-Hamici, Z., Weidmann, H., Blum, Y., Proust, C., Durand, H., Iannacci, F., Codoni, V., Gaignard, P., Thérond, P., Civelek, M., Karabina, S.A., Lusis, A.J., Cambien, F., Ninio, E., 2016. Role of lipid phosphate phosphatase 3 in human aortic endothelial cell function. Cardiovasc Res 112, 702–713. https://doi.org/10.1093/cvr/cvw217
-
Bissonnette, L., Drissennek, L., Antoine, Y., Tiers, L., Hirtz, C., Lehmann, S., Perrochia, H., Bissonnette, F., Kadoch, I.-J., Haouzi, D., et al. (2016). Human S100A10 plays a crucial role in the acquisition of the endometrial receptivity phenotype. Cell Adhesion & Migration 10, 282–298.
-
Alvarado-Ruiz, L., Martinez-Silva, M.G., Torres-Reyes, L.A., Pina-Sanchez, P., Ortiz-Lazareno, P., Bravo-Cuellar, A., Aguilar-Lemarroy, A., and Jave-Suarez, L.F. (2016). HOXA9 is Underexpressed in Cervical Cancer Cells and its Restoration Decreases Proliferation, Migration and Expression of Epithelial-to-Mesenchymal Transition Genes. Asian Pacific Journal of Cancer Prevention 17, 1037–1047.
-
Zhang, R.-Y., Yu, Z.-H., Zeng, L., Zhang, S., Bai, Y., Miao, J., Chen, L., Xie, J., and Zhang, Z.-Y. (2016). SHP2 phosphatase as a novel therapeutic target for melanoma treatment. Oncotarget.
-
Cormier, N., Yeo, A., Fiorentino, E., and Paxson, J. (2015). Optimization of the Wound Scratch Assay to Detect Changes in Murine Mesenchymal Stromal Cell Migration After Damage by Soluble Cigarette Smoke Extract.. Journal of Visualized Experiments.
-
Leach, D.A., Need, E.F., Toivanen, R., Trotta, A.P., Palenthorpe, H.M., Tamblyn, D.J., Kopsaftis, T., England, G.M., Smith, E., Drew, P.A., et al. (2015). Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome. Oncotarget 6, 16135–16150.
-
Nathalia S. Laszkiewicz, Graziela G. Romagnoli, Carolina M. Gorgulho, Ethel Cesarman, Ramon Kaneno, Deilson E. Oliveira. In vitro migration and cellular invasion of human cells expressing variants of the Epstein-Barr virus LMP1 oncoprotein. In Nathália Suiti Laszkiewicz (2015). Migração e invasão celular in vitro de células humanas expressando variantes da proteína LMP1 do vírus de Epstein-Barr (EBV). Mestre em Patologia. UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO.”
-
Sánchez-Bailón, M.P., Calcabrini, A., Mayoral-Varo, V., Molinari, A., Wagner, K.-U., Losada, J.P., Ciordia, S., Albar, J.P., and Martín-Pérez, J. (2015). Cyr61 as mediator of Src signaling in triple negative breast cancer cells. Oncotarget 6, 13520–13538.
-
Sushobhna Batra, Richard A. Rabin Presentation: Effects of Ethanol on Brain Injury: Role of Microglial Migration. 2015, University at Buffalo
Other references
-
Palacio, J., Johanna, L., n, G., Pablo, J., Carolina, D., and Carlos, J. ENSAYO DE CICATRIZACIÓN IN VITRO, PARA EVALUAR MIGRACIÓN CELULAR v2. protocols.io.kdics4e.
-
Protocolo de ENSAYO DE HERIDA (Spanish), Universidad Autonoma de Madrid, 2013
Literature
[1] Jonkman, J.E.N., Cathcart, J.A., Xu, F., Bartolini, M.E., Amon, J.E., Stevens, K.M., and Colarusso, P. (2014). An introduction to the wound healing assay using live-cell microscopy. Cell Adhesion & Migration 8, 440–451.