Chemistry - Meadeee/GAMSAT-2021 GitHub Wiki
Chemistry
Naming Molecular Compounds
Atom and molecules

The Periodic Table

Bonds

Chemical Bonds
Hydrogen Bonds
Ionic
Covalent Bonds

Equilibria, Le Chatelier’s principle and its applications
Thermodynamics – 1st law of thermodynamics, energy, pressure, entropy
Acids and bases
- An Arrhenius acid is any species that increases the concentration of H+ in aqueous solution.
- An Arrhenius base is any species that increases the concentration of **OH- **in aqueous solution.
- In aqueous solution H+ ions immediately react with water molecules to form hydronium ions H3O
- In an acid-base or neutralization reaction, an Arrhenius acid and base usually react to form water and a salt.
From the vinegar in your kitchen cabinet to the soap in your shower, acids and bases are everywhere! But what does it mean to say that something is acidic or basic? In order to answer this question, we need to examine some of the theories describing acids and bases. In this article, we will focus on the Arrhenius theory.
Electrolysis

Lewis Dot Structures
Multiple Bonds
Resonance
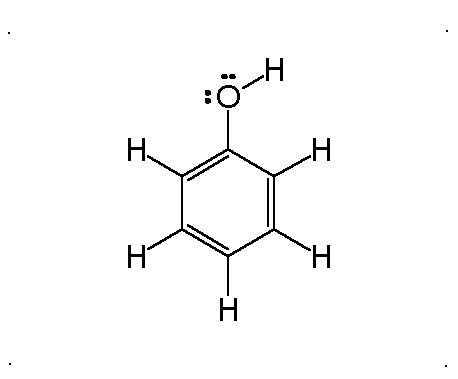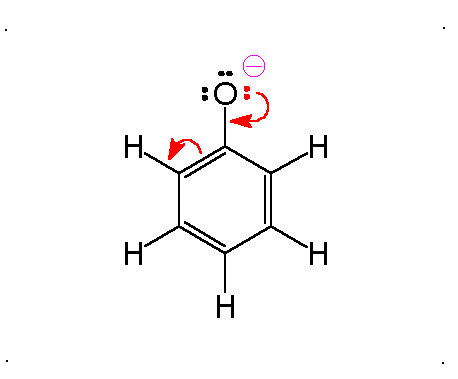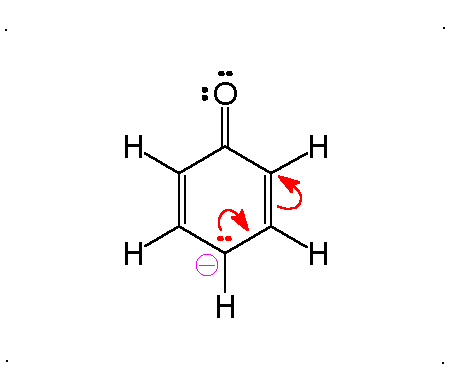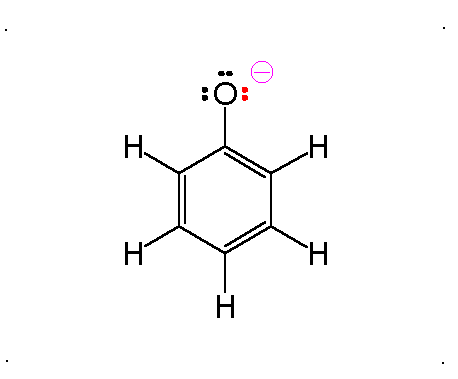
Molecular Polarity
Hybridization
Hybrid Orbitals
Gases
Avogadro’s Law, Equation of State
Graham’s Law
Liquids
Maxwell’s Distribution Plot

Boiling points and Melting Points
Le Chatelier’s Principle
Solutions and Phase Diagrams
Raoult’s Law
Titrations
