Lab 3 Project Kit: Calorimeter - MAE221/Thermodynamics-Lab GitHub Wiki
Calorimetry "is the science of measuring the change of energy involved in a chemical process." Calorimetry is the measurement of heat, and calorimeters are instruments for measuring heat.
One simple type of constant pressure calorimeter is the "coffee cup" calorimeter which can be made at home but is not very accurate. The lab on campus contains a type of constant volume calorimeter called a bomb calorimeter, which is much more accurate but also more complicated. There are many other types of calorimeters such as isothermal calorimeters, which use phase transitions to maintain a constant temperature while absorbing heat. you will be using a food calorimeters from Eisco Labs for your experiments at home. This calorimeter consists of a flask suspended above a food stand in a metal enclosure.


Eisco Labs food calorimeter

bomb calorimeter
We cannot measure heat directly. The bomb and food calorimeters operate by measuring the change in temperature of a fluid that results from the flow of heat we are interested in. Assuming there is no phase change in the operating fluid, the heat is related to this temperature change by the specific heat equation:
Specific Heat Equation
where
Many calorimeters use water as the operating fluid because it has a higher specific heat than most other common substances (1 calorie/gram at standard conditions). Keep in mind that the specific heat of any substance is typically a function of temperature, but we can often approximate it as constant if our range of temperatures is modest.
For a more detailed overview on the theory of a bomb calorimeter, checkout this textbook chapter.
-
Setup the Princeton VPN
-
Install the Amcrest Chrome app
-
In the Chrome browser, navigate to: chrome://apps/
-
Launch the “Amcrest Web View” app
-
In Amcrest Web View, enter the remote camera's IP address: http://cee4.princeton.edu/
-
Login: User = admin Password = cee102b4
-
You may have to go into the “Setup” tab to do trial-and-error with the “Mirror” and “Flip” settings to get the right image orientation and pan-tilt-zoom response.
Here is the calibration data for the bomb calorimeter taken during lab: Tuesday
Here is the calibration data for the bomb calorimeter taken during lab: Thursday
The thermocouple circuit that you used in Lab 1 Part 2 should be adequate for this project. However, you will need to edit the MATLAB code from that lab to continuously record temperatures. Even though we only need the initial and maximum temperatures, it will be helpful to have the full time history in order to properly identify the peak temperature. Remember to reference the MATLAB Tutorial for help with editing the code.
If you are experiencing issues with your Photon or thermocouple, try using this circuit instead:
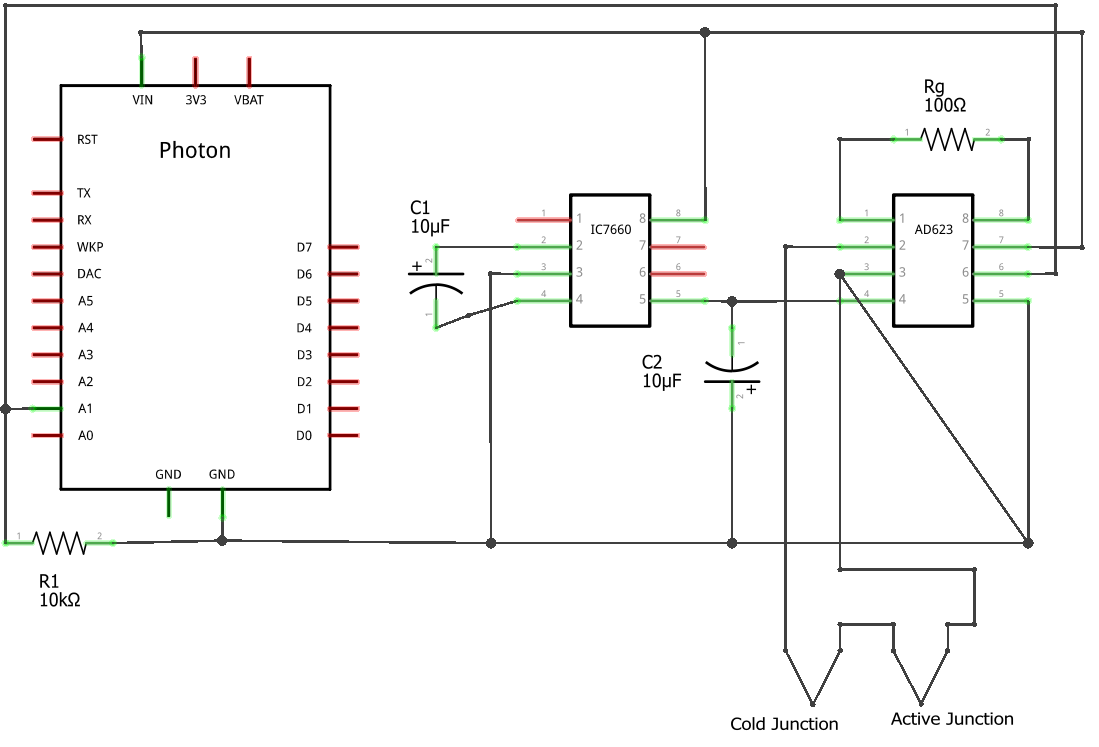
Thermocouple Circuit
NOTE: Gain will be 1000. Please adjust your code to take this into account.
- Calibrate your temperature instrumentation before taking measurements.
- Dry samples with large surface area are the most flammable. If you have trouble igniting your sample, try drying it in an oven and pulverizing it.
- You don’t need to place your samples on the cork & nail that comes with the calorimeter. You can use a glass/metal/ceramic dish or make your own stand out of non-flammable materials.
- Ensure good ventilation for combustion so that you burn as much of your sample as possible.
- You can adjust the temperatures range you expect to measure by use more or less water in your flask.
- Repeat experiments several times to get better statistical measurements.
- You can consider making modifications to your calorimeter to try and improve the measurements.
Perform your experiments in a clean environment that is free of flammable materials. It is recommended to cover your work space with a sheet of aluminum foil to protect your table and make clean-up easier. Start testing with small samples to prevent large fires, then you can increase the sample size as needed.
If you burn yourself, practice basic first aid. Cool the burned area under running water or apply a cold compress to the burned area. Please be smart about playing with fire!