Coral Bacteria Culturing - IBEMicrobeHub/culturing GitHub Wiki
Background
Culturing bacteria has been done for nearly 200 years, having adjustments from liquid medias, semi-solid medias, to solid medias. In 1860, the first liquid artificial culture medium was created by Louis Pasteur. The advancement of solid agar wasn't until 1881 when Robert Koch presented his new isolation technique at the International Medical Congress in London. After recognizing the difficulties of utilizing broth medias, he began looking for an alternative. The first real agar was made by combining a meat extract with gelatin. This new gelatinous material was poured onto glass plates before being inoculated and placed under a bell jar. Solid media has since evolved when Fannie Hesse suggested using agar instead of gelatin in 1882. Due to its higher melting point, clearer color, and resistance to digestion by bacterial enzymes, agar advanced the quality of solid media immensely. Corals hold mutualistic relationships with many microorganisms including fungi, viruses, bacteria, and archaea. This consortium is termed the coral holobiont. The interactions between the host and its inhabitants is crucial to the fitness and survivability of the coral. The bacterial symbionts are involved in aspects of the coral biology, including defense, growth, survival, nutrition, and general health.
While culturing is essential to broadening knowledge about coral microbiomes, there are restrictions including the "Great Plate Anomaly". This term was given to the idea that only a fraction of bacteria is currently able to be cultured on medias available today. This is due to temperature, pH, nutrition, etc. requirements of certain strains of bacteria. It is not that the culturing is impossible, there is currently not enough known in addition to the complexity of cross-feeding systems between host-associated microorganisms. Additionally, many environmental bacteria are outcompeted by copiotrophic bacteria on common nutrient-rich media. In order to combat this, novel medias can be made to retrieve a higher level of diversity of bacteria.

Phylum (a), order (b), and genus (C) level profiles of coral-associated bacteria isolated from each type of culture medium. Taxa (i.e., orders and genera) representing less than 1% of the total percentage of isolates were pulled together and classified as “Others." (Sweet et al., 2021)
Methods
Airbrushing Corals
1. Obtain 2 inch of coral fragments 2in of 2 ACER fragments 2in of 2 OFAV fragments
2. Gently airbrush one coral at a time, collecting tissues in separate bags
Creating Coral Tissue Agar
1. Add 10mL of coral tissue to a 1000mL flask
2. Add 390mL of PBS 1x to the same 1000mL flask
3. Add 7.5g of Agarose to the same 1000mL flask
4. Add magnetic stir bar and agitate on hot plate for 5 minutes.
Heat: 10
Stir: 8
5. Once completely mixed, cover top of 100mL flask with tin foil and add 2 lines of autoclave tape
6. Fill bottom of plastic bin with 1 inch of water
7. Place flask in bin and set in autoclave
Liquid Autoclave 15 minutes
8. Using hot gloves, rinse water out of bin
9.* Pour agar into approximately 24 plates, only covering bottom
Duration to cure: 15 minutes
10 Store plates upside down in 4 degrees Celsius fridge
Creating 1/2 Conc. Marine Agar
1. Tare weight scale with a weigh boat to 0.0g
2. Add 18.7g of Difco Marine Agar to the same 1000mL Flask
4. Add 500mL of DI water to the same 100mL Flask
4. OPTIONAL Add magnetic stir bar and agitate on hot plate for 5 minutes.
Heat: 10
Stir: 8
6. Once completely mixed, cover top of 1000mL flask with tin foil and add 2 lines of autoclave tape
7. Fill bottom of plastic bin with 1 inch of water
8. Place flask in bin and set in autoclave
Liquid Autoclave 15 minutes
9. Using hot gloves, dump water out of bin
10.* Pour agar into approximately 24 plates, only covering bottom
Duration to cure: 15 minutes
When pouring plates, work near a flame. The opened flask sound stay within 2cm of the flame at all times. Do not wear gloves for risk of burning, keep area and hands as sterile as possible.
11 Store plates upside down in 4 degrees Celsius fridge Stack in plate sleeve and label with date, media, and initials.
Creating TCBS Agar
1. Tare weight scale with a weigh boat to 0.0g
2. Add 44.54g of TCBS to 1000mL Flask
3. Add 500mL of DI water to the same 100mL Flask
4. Add magnetic stir bar and agitate on hot plate for 5 minutes.
Heat: 10
Stir: 8
5. Once completely mixed, cover top of 100mL flask with tin foil and add 2 lines of autoclave tape
6. Fill bottom of plastic bin with 1 inch of water
7. Place flask in bin and set in autoclave
Liquid Autoclave 15 minutes
8. Using hot gloves, rinse water out of bin
9.* Pour agar into approximately 24 plates, only covering bottom
Duration to cure: 15 minutes
When pouring plates, work near a flame. The opened flask sound stay within 2cm of the flame at all times. Do not wear gloves for risk of burning, keep area and hands as sterile as possible.
10 Store plates upside down in 4 degrees Celsius fridge Stack in plate sleeve and label with date, media, and initials.
Creating Culture Lawns
1. Turn on UV Radiation in aerated hood to sterilize (approx. 5 min)
2. Wipe down aerated hood with 70% ethanol
3. Light flame under hood
4. Pipette 2 uL of coral tissue previously collected onto plate
Make sure not to bump into the bag or hood, if it happens, discard tip and start over
5. Using "Tongue scraper", spread liquid tissue across the plate. Create 1 replicate of each lawn. Refer to image below
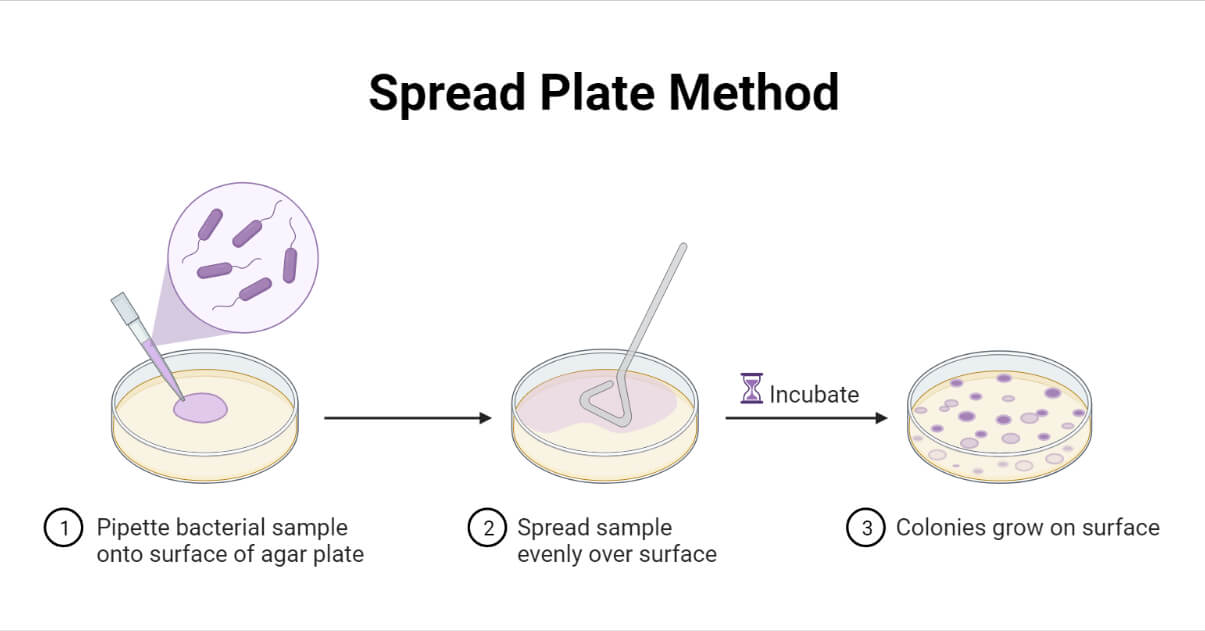
6. Store in incubator to let the colonies grow
7. Wipe down all surfaces used with 70% ethanol
8. Turn on UV Radiation in aerated hood (approx. 5 minutes)
Isolating Colonies
1. Turn on UV Radiation in aerated hood (approx. 5 min)
2. Wipe down aerated hood with 70% ethanol
3. Light flame under hood
4. Heat inoculation loop in flame for 5 seconds and dip it in the daughter plate to cool off
Close the lid of the daughter plate immediately and move onto next step quickly to avoid contamination
5. Remove lid of parent plate and lightly collect the desired sample before immediately closing the lid
Try to isolate a SINGLE colony
6. Remove lid of daughter plate and make 4-5 lines gently across the surface of the agar before immediately closing the lid
Refer to the below image regarding steps 6-11

7. Heat inoculation loop in flame for 5 seconds and gently waft in air to cool for 5 seconds
8. Open the daughter plate, starting where you left off with your last lines, gently make 4-5 lines gently across the surface of the agar perpendicular to the direction you originally went and immediately close the lid
Heat inoculation loop in flame for 5 seconds and gently waft in air to cool for 5 seconds
The purpose of taking from the previous spread is to better isolate the sample to single colonies
9. Open the daughter plate, starting where you left off with your last lines, gently make 4-5 lines gently across the surface of the agar perpendicular to the direction you originally went and immediately close the lid
Heat inoculation loop in flame for 5 seconds and gently waft in air to cool for 5 seconds
10. Open the daughter plate, starting where you left off with your last lines, gently make 4-5 lines gently across the surface of the agar perpendicular to the direction you originally went and immediately close the lid
Make sure the end of your strokes do not tough your initial strokes
Heat inoculation loop in flame for 5 seconds and gently waft in air to cool for 5 seconds
11. Open the daughter plate, starting with the last strokes, light squiggle the sample down, making sure not to touch any other previous strokes and immediately close the lid
Refer to above image
12. Heat inoculation loop and put lid on flame before wiping down work space with 70% ethanol
13. Turn on UV Radiation in the aerated hood
14. Put plates into incubator at 25 degrees Celsius
Liquid Suspension (eg. Marine Broth)
1. Suspend 18.7g of Marine Broth powder in 500mL of purified water in a 1000mL flask
2 Place magnetic spinner in the same flask and stir while heating to a boil
3 After boiling, cover top in tin foil and 2 lines of autoclave tape
4 Fill plastic bin with about 1 inch of water to cover bottom of bin before placing flask into
5 Autoclave
Liquid Autoclave 15 minutes
6 Label 21 culture tubes
7 Add 10mL of marine broth to each culture tube
8 Heat up inoculation loop with flame for 7 seconds and wave for 5 seconds to cool with inoculation loop before dipping into culture tube once and close tube
Make sure that the inoculation loop does not touch the sides
Make sure the cap is on AERATED mode
9 Clean up loop and area with 70% ethanol and place tubes in incubator
Glycerol Freezing
1. Add 500 μL of the overnight liquid culture to 500 μL of 50% glycerol in a 2 mL screw top tube or cryovial and gently mix
Make the 50% glycerol solution by diluting 100% glycerol in dH20
Snap top tubes are not recommended as they can open unexpectedly at -80°C
2. Freeze the glycerol stock tube at -80°C